Research examples
Controlling surface composition
Whilst simple monometallic catalysts are effective for a number of processes you have limited ability to tune the geometric and electronic properties of the working catalyst. In a bimetallic system, the geometric arrangement is linked to surface structure (i.e., how the two metals are arranged on the surface of a single particle). The electronic properties are then linked to both the surface and near surface atoms. In a recent piece of work, we explored CuPd bimetallics for acetylene and propyne hydrogenation. Since the two metals alloy across the full range of compositions it was possible adjust the surface composition by simply changing the proportion of the two metals. At the ‘sweet spot’ the catalyst had a small number of Pd surface atoms yet showed high activity. From these results, it was inferred that Pd may be the active site for hydrogen dissociation. Hydrogen adatoms would the spillover onto neighbouring Cu atoms allowing for the reaction to occur on a Cu surface. Interestingly, this happened at a temperature far lower than you would normally expect to occur on a monometallic Cu catalyst. The concept is depicted in the image below:
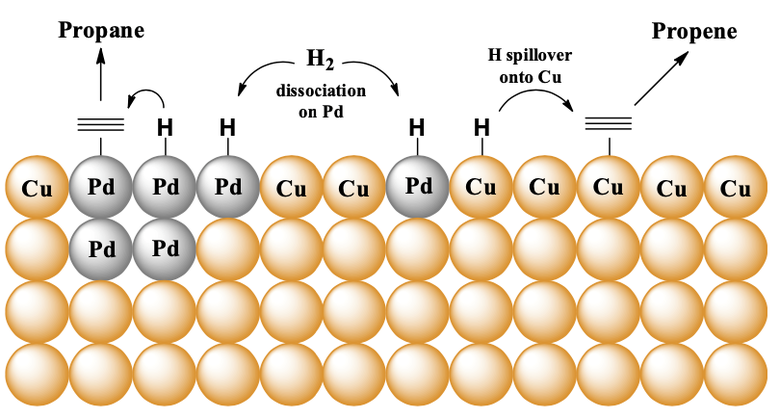
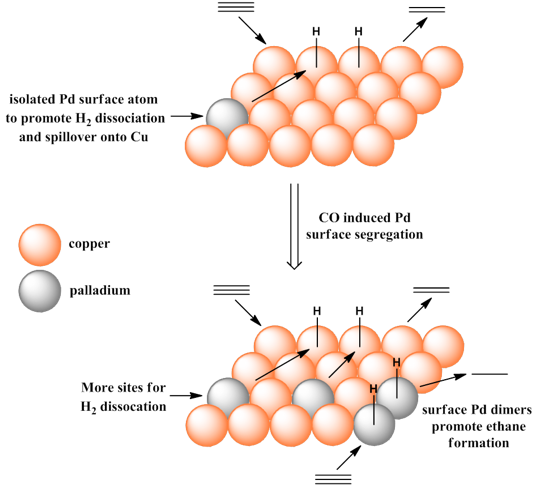
Whilst changing the proportions of the two metals is a simple way to alter surface composition it does rely upon carefully controlled catalyst preparation. In other words, if your preparation method is not reproducible then you can’t amend the surface composition post synthetically. In the case of a CuPd bimetallic, it is possible to alter surface composition by adsorbate induced surface segregation. This concept is based around the fact that CO adsorbs more strongly to Pd than Cu. Therefore, by heating a bimetallic in the presence of CO, Pd atoms are pulled towards the surface. We used this concept to increase the activity of a CuPd bimetallic without adding additional Pd to the composition. It was also possible to correlate surface Pd species with selectivity (i.e., Pd dimers favour over-hydrogenation). This concept is depicted below:
A. J. McCue, C. J. McRitchie, A. M. Shepherd and J. A. Anderson. Cu/Al2O3 catalysts modified with Pd for selective acetylene hydrogenation. Journal of Catalysis 319, 2014, 127-135.
A. J. McCue, A. Gibson & J. A. Anderson. Palladium assisted copper/alumina catalysts for the selective hydrogenation of propyne, propadiene and propene mixed feeds. Chemical Engineering Journal, 295, 2016, 384-391.
A. J. McCue & J. A. Anderson. CO induced surface segregation as a means of improving surface composition and enhancing performance of CuPd bimetallic catalysts. Journal of Catalysis 329, 2015, 538-546.
Metal Chalcogenides
For a number of years, we have also been exploring the use of metal chalcogenides (i.e., palladium sulfide) for alkyne hydrogenation. This type of system is interesting to study because the structure is very well defined and based on composition (i.e., PdS, Pd16S7 and Pd4S). In other words, the geometric arrangement of the two components is well known. Within our research, we have demonstrated that the Pd4S phase is both exceptionally active and selective for acetylene hydrogenation with performance rivalling the best reported catalysts in literature. Through a combination of detailed characterisation methods and theoretical calculations, the exceptional performance of the Pd4S phase is attributed to a geometric arrangement where S atoms act as spacers around the Pd atoms to create a particular geometry. The electronic effect of coupling Pd with a more electronegative element then favours product desorption and enhances selectivity. This concept is depicted below:
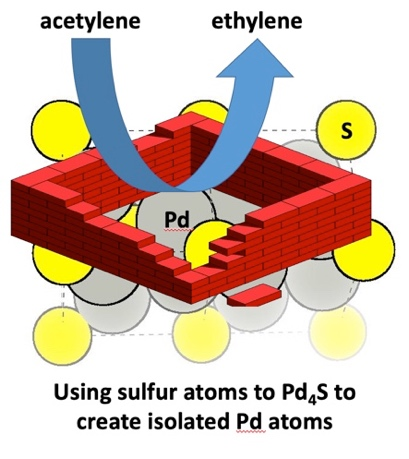
A. J. McCue, A. Guerrero-Ruiz, I. Rodriguez-Ramos and J. A. Anderson. Palladium sulfide – a highly selective catalyst for the gas phase hydrogenation of alkynes to alkenes. Journal of Catalysis 340, 2016, 10-16.
A. J. McCue, A. Guerrero-Ruiz, C.Ramirez-Barria, I. Rodriguez-Ramos and J. A. Anderson. Selective hydrogenation of mixed alkyne/alkene streams at elevated pressure of a palladium sulfide catalyst. Journal of Catalysis 335, 2017, 40-52.
Y. Liu, A. J. McCue, J. Feng, S. Guan, D. Li and J. A. Anderson. Evolution of palladium suklfide phases during thermal treatments and consequences for acetylene hydrogenation. Journal of Catalysis 364, 2018, 204-215.
Y. Liu, Y. Li, J. A. Anderson, J.Feng, A. Guerrero-Ruiz, I. Rodriguez-Ramos, A. J. McCue and D. Li. Journal of Catalysis 383, 2020, 51-59.
