
Advanced Research Fellow
- About
-
- Email Address
- hajime.murakami1@abdn.ac.uk
- Office Address
- School/Department
- School of Medicine, Medical Sciences and Nutrition
- Research
-
Research Overview
Production of gametes (eggs and sperm) involves massive genome fragmentation followed by accurate resealing of the genome, in the process known as meiotic recombination. How do cells complete this seemingly reckless meiotic recombination process without mishap? Our research focuses on the initiation stage of meiotic recombination: the programmed formation of DNA double-strand breaks (DSBs), catalysed by ‘DSB proteins’. We aim to define the molecular framework regulating DSB proteins binding along meiotic chromosomes to delineate fundamental principles creating robust meiotic recombination.
Sexual reproduction for diversity
Life begins when an egg and a sperm from parents merge into a single cell, the fertilised egg, which multiplies into many cells to create our body. Sperm and egg each contribute 23 chromosomes, making a total of 46 chromosomes in the fertilised egg. When our body produces sperm or eggs, cells undergo a special cell division called meiosis, which halves the number of chromosomes (Fig. 1). Chromosomes consist of long stretches of DNA, which encode the many genes required to make our body. When meiosis occurs, the chromosomes inherited from the mother are reshuffled with those from the father, forming a completely new set of chromosomes with a unique combination of genes. This reshuffling process diversifies gene combinations from generation to generation, creating evolutionary adaptability to environmental changes.
The process of reshuffling the genes in the chromosomes is called recombination. Recombination operates by cutting DNA and exchanging sections of the DNA between maternal and paternal chromosomes. If recombination fails, the wrong number of chromosomes, or incorrectly assembled chromosomes, can be passed into sperm and eggs. This type of fault is a leading cause of miscarriage and congenital syndromes including Down, Edward, Patau (trisomy 21, 18, 13) & Turner (monosomy X). Overall, about 0.3% of newborn infants have an incorrect chromosome number. Given its importance, organisms are equipped with various mechanisms to ensure correct meiotic recombination. We are investigating how meiotic recombination is controlled. For this research we use yeast cells, which undergo meiosis just like humans, and are easy to manipulate experimentally.
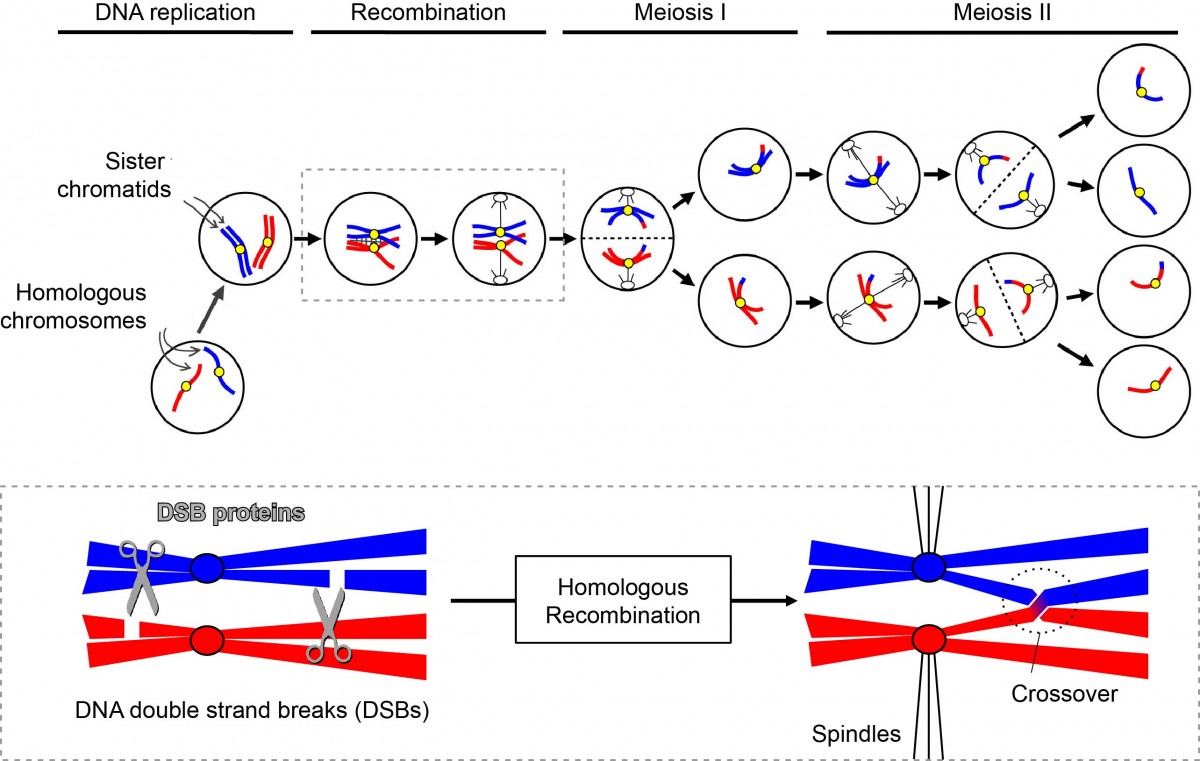
Figure 1. Recombination is necessary for chromosome segregation during meiosis. In meiosis, chromosomes are replicated once and then segregated twice to halve the number of chromosomes. Before the first chromosome segregation, meiotic cells cleave chromosomal DNA to induce homologous recombination. Meiotic recombination yields reciprocal exchanges of arms between homologous chromosomes. These crossovers between homologs (chiasmata) are vital for proper attachment of spindles and segregation of homologous chromosomes. DNA scissors and their manager in meiosis
When meiosis begins, cells make a group of proteins that act together as scissors to cut the DNA for recombination (Fig. 1, bottom). To produce normal sperm and eggs, these DNA scissors need to be well controlled to prevent recombination failures, but the control mechanisms are still enigmatic. Recent findings have led us to hypothesise that proteins called Hop1 and Red1 direct the activity of these scissors, telling them where, when, and how often to cut DNA.
Hop1 and Red1 are part of the 'chromosome scaffold' of meiotic cells and are believed to help the scissors bind to chromosomes (Fig. 2). We discovered further roles for Hop1 and Red1 in the process. Paradoxically, even though their main role is to promote the recruitment of DNA scissors to chromosomes, we found that Hop1 and Red1 can prevent scissors from randomly binding along chromosomes. Moreover Hop1 and Red1 are needed not just to recruit but also to activate the scissors for cutting DNA. Also, Hop1 and Red1 pay extra attention to short chromosomes, preferentially directing the DNA scissors to short chromosomes to ensure they get cut. Overall I propose that these two 'manager proteins' Hop1 and Red1 direct the scissors to chromosomal locations favourable for correct recombination, while simultaneously preventing the scissors from cutting at dangerous places, both by excluding them from such locations and by allowing the scissors to cut DNA only when both manager proteins are present.
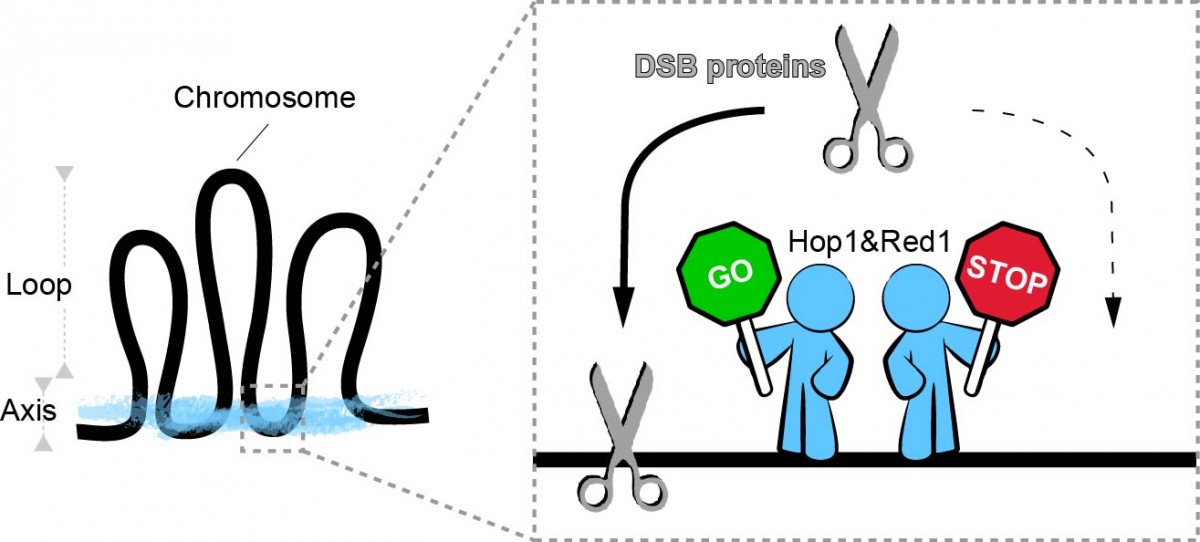
Figure 2. Meiotic chromosome axis proteins (Hop1 and Red1) organize the chromosome in an array of loops tethered at the base. We found that axis proteins determine the spatial pattering of DSB proteins along chromosomes including the preference for short chromosomes, and also activate DSB proteins for cleavage. Current Research
We are investigating these new functions of Hop1 and Red1 to test our hypothesis. We will elucidate how these proteins behave in meiotic cells to understand the molecular mechanisms that control recombination. Since these scissors and manager proteins are present in human and yeast, new knowledge from this study will advance our understanding of how our eggs and sperm are made and how their production process can fail. This work will therefore make critical contributions to the understanding of fertility and the formation of new genomes and, to the development of reproductive medicine. For example, once the risk of chromosome abnormalities due to mutations in these manager genes is understood, it will help patients to select the best fertility treatments. Such understanding may also help design strategies to avoid faulty meiosis and consequent health problems completely.
Research Areas
Accepting PhDs
I am currently accepting PhDs in Biomedical Sciences.
Please get in touch if you would like to discuss your research ideas further.
Research Specialisms
- Genetics
- Genomics
- Molecular Biology
Our research specialisms are based on the Higher Education Classification of Subjects (HECoS) which is HESA open data, published under the Creative Commons Attribution 4.0 International licence.
Past Research
Mu, X, Murakami, H*, Mohibullah, N & Keeney, S. Chromosome-autonomous feedback downregulates meiotic DNA break competence upon synaptonemal complex formation. Genes & Development, doi:10.1101/gad.342873.120 (2020).
This article describes the molecular mechanisms of a DSB feedback pathway, in which synaptonemal complex formation caused by successful recombination removes a DSB protein.
Murakami, H*, Lam, I, Huang, PC, Song, J, van Overbeek, M & Keeney, S. Multilayered mechanisms ensure that short chromosomes recombine in meiosis. Nature 582, 124-128, doi:10.1038/s41586-020-2248-2 (2020).
This article reports multiple pathways that ensure all chromosomes recombine in meiosis. One of these pathways controls duration of the DSB-competent state, which represents a novel concept, and implies that cis acting sequence boosts DSBs on specific chromosomes.
Murakami, H & Keeney, S. Temporospatial Coordination of Meiotic DNA Replication and Recombination via DDK Recruitment to Replisomes. Cell 158, 861-873, doi:10.1016/j.cell.2014.06.028 (2014).
This article reports an elegant molecular mechanism that temporally coordinates DNA replication and meiotic DSB formation, showing that an initial step towards DSB formation occurs only on replicated chromatin, because a key kinase is sequestered at replication forks.
Funding and Grants
Medical Research Council (MRC) Career development award
- Publications
-
Page 2 of 2 Results 11 to 13 of 13
Meiotic association between Spo11 regulated by Rec102, Rec104 and Rec114
Nucleic Acids Research, vol. 35, no. 4, pp. 1119-1133Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1093/nar/gkl1162
- [ONLINE] View publication in Scopus
Correlation between premeiotic DNA replication and chromatin transition at yeast recombination initiation sites
Nucleic Acids Research, vol. 31, no. 4, pp. 4085-4090Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1093/nar/gkg441
- [ONLINE] View publication in Scopus
Targeted stimulation of meiotic recombination
Cell, vol. 111, no. 2, pp. 173-184Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/S0092-8674(02)01002-4
- [ONLINE] View publication in Scopus
