
Chair in Development Neurobiology
- About
-
- Email Address
- l.erskine@abdn.ac.uk
- Telephone Number
- +44 (0)1224 437324
- Office Address
Room 4:18
Institute of Medical Sciences
School Of Medicine, Medical Sciences and Nutrition
University of Aberdeen
Foresterhill
Aberdeen
AB25 2ZD
- School/Department
- School of Medicine, Medical Sciences and Nutrition
Biography
I am a developmental biologist with a particular interest in visual system development. I obtained my PhD at the University of Aberdeen studying the role of electric fields in directing nerve outgrowth. This was followed by a postdoctoral position at University College London investigating the mechanisms of neuronal cell fate determination. Funded by EMBO and HFSPO Long-term Fellowships, I did a second postdoctoral Fellowship at Columbia University, USA studying the mechanisms controlling axon guidance in the developing optic pathway. This work formed the foundation for my current focus on visual system development. In 2001 I was appointed as a Lecturer at UCL’s Institute of Ophthalmology. I moved to the University of Aberdeen as a Senior Lecturer in 2007 and was appointed to Professor in 2012. As well as leading a research team studying the cellular and molecular mechanisms controlling visual system development, I am actively involved in teaching. I am coordinator for human embryology and developmental biology courses at both undergraduate and postgraduate level and Co-Programme coordinator for an MSc Programme in Reproductive and Developmental Biology.
My work has identified a number of key molecules important for patterning the binocular visual pathways and normal wiring of the visual brain, including ephrinB2, Robos/Slits, NrCAM, VEGF-A/NRP1 and DSCAM. Related studies have advanced our understanding of the role of disease associated molecules in control of retinal cell survival, proliferation, organisation and function. A current major focus of my group’s work is the mechanisms controlling development of the anterior part of the eye, in particular the ciliary body, iris and trabecular meshwork. This work is aimed at understanding the relationship between events in early eye development and later risk for common ocular conditions such as glaucoma, myopia (short-sightedness) and hyperopia (long-sightedness).
Qualifications
- BSc (1st Class) Medical Science1991 - University of Aberdeen
- PhD Physiology1994 - University of Aberdeen
Memberships and Affiliations
- Internal Memberships
-
- Elected Member University Senate, 2018 -
- Co-Lead Molecule Medicine Programme, 2018 -
- IMS Athena Swan Self-Assessment Team/Equality, Diversity and Inclusion Group, 2016 -
- Academic Lead Microscopy and Histology Facility, 2016 -
- Co-lead Cell, Developmental and Cancer Biology Programme, 2015 - 2018
- Academic Line Manager, 2015 -
- External Memberships
-
- Reviewing Editor eNeuro, 2019 –
- Member Fight For Sight Grant Assessment Panel, 2019-
- External Examiner King’s College London BSc and iBSc Neuroscience and Anatomy and Human Sciences Degree Programmes, 2012 -2016
- Member of British Society For Developmental Biology (BSDB) Committee, 2010-2015
- Member organising committee for Axon 2017 (Meeting on Axon Guidance, Circuit Development and Regeneration; Klosterneuburg, Austria), 2017
- Member organising committee for Axon 2015 (Meeting on Axon Guidance, Circuit Development and Regeneration; Klosterneuburg, Austria), 2015
- Member organising committee for Joint Annual Meeting of British Society for Cell Biology (BSDB) and British Society of Cell Biology (BSCB), Warwich University UK, 2014
- Member organising committee Autumn Meeting of British Society for Cell Biology (BSDB) on Mechanisms of Axon Guidance and Regeneration, Aberdeen UK, 2013.
- Organiser Winter Meeting of Scottish Developmental Biology Group, 2008 and 2011.
Latest Publications
CXCL12 promotes the crossing of retinal ganglion cell axons at the optic chiasm
Development, vol. 151, no. 2, dev202446Contributions to Journals: ArticlesKnockdown of Slit signalling during limb development leads to a reduction in humerus length
Developmental Dynamics, vol. 250, no. 9, pp. 1340-1357Contributions to Journals: ArticlesWiring the Binocular Visual Pathways
International Journal of Molecular Sciences, vol. 20, no. 13, 3282Contributions to Journals: Review articles- [ONLINE] DOI: https://doi.org/10.3390/ijms20133282
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/12673/1/ijms_20_03282.pdf
- [ONLINE] View publication in Mendeley
A Retino-retinal Projection Guided by Unc5c Emerged in Species with Retinal Waves
Current Biology, vol. 29, no. 7, pp. 1149-1160Contributions to Journals: ArticlesGuidance of retinal axons in mammals
Seminars in Cell and Developmental Biology, vol. 85, pp. 48-59Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/j.semcdb.2017.11.027
- [ONLINE] View publication in Scopus
- [ONLINE] View publication in Mendeley
- [ONLINE] View publication in Scopus
Prizes and Awards
Suffrage Science (Women in Science) Award by MRC Clinical Sciences Centre, 2014
- Research
-
Research Overview
Work in my lab is focused on understanding the cellular and molecular mechanisms regulating early visual system development and the importance of events that occur pre-birth for later risk of eye disease.
We are studying the mechanisms controlling the development of the anterior part of the eye (cornea, ciliary body, iris and trabecular meshwork) and the importance of anterior eye development for establishment of eye size and as a later risk factor for common, potentially blinding eye disorders such as glaucoma and myopia. Of particular interest is the role of the lens as an essential signalling centre instructing anterior eye development.
Another ongoing focus of our work is the is the cellular and molecular mechanisms that guide retinal axons from the eye to target regions in the brain. Of particular interest is the signalling mechanisms that constrain axons to their correct path, enabling appropriate connections to form between the eye and the brain, and pattern the binocular visual pathways enabling stereopsis. Defects in the development of functional connections between the eye and the brain can result in severe visual impairment and blindness. Understanding how these connections form normally also is important for formulation of strategies aimed at restoring vision following degeneration or damage of the optic nerve.
In collaboration with Professor Neil Vargesson’s group (https://www.abdn.ac.uk/ims/research/profiles/n.vargesson) we also are investigating the mechanisms underlying the teratogenic effect of drugs such as thalidomide and Primidos on embryonic development.
Research Areas
Accepting PhDs
I am currently accepting PhDs in Biomedical Sciences.
Please get in touch if you would like to discuss your research ideas further.
Research Specialisms
- Genetics
- Neuroscience
- Molecular Biology
- Cell Biology
- Developmental Biology
Our research specialisms are based on the Higher Education Classification of Subjects (HECoS) which is HESA open data, published under the Creative Commons Attribution 4.0 International licence.
Current Research
ANTERIOR EYE DEVELOPMENT:
Events in early eye development can have a profound influence on visual function. However, the relationship between events in the early eye and later risk of eye disease is poorly understood. A major focus of the ongoing work in my lab is aimed at unravelling the mechanisms that control anterior eye development in order to understand the basis of potentially blinding conditions, such as anophthalmia and microphthalmia, and factors that contribute to common ocular disorders such as myopia (short-sightedness), hyperopia (long-sightedness) and glaucoma.
In all vertebrate species, including chicken, mouse, fish and humans, the presence of a normal living lens is essential for growth and patterning of the developing eye. In the absence of a normal lens, eye size is impaired, the neural retinal becomes highly folded in the vitreal cavity and anterior segment of the eye (ciliary body, iris, cornea, trabecular meshwork) develops abnormally.
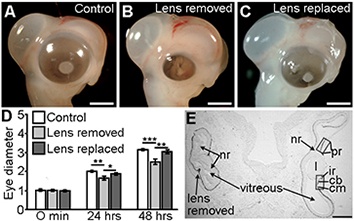
Figure 1. The lens control eye development and size.
We have demonstrated that the lens acts indirectly to control eye size through controlling patterning and function of the developing peripheral optic cup (future iris and ciliary body). Lens-regulated genes with a central role in controlling eye development and size include factors essential for retinoic acid synthesis (e.g. RDH10), transcription factors such as OTX1, and members of the BMP and WNT signalling families. Our work has demonstrated that through modulating ciliary body development and function, lens-dependent signalling pathways control secretion of vitreal proteins and, consequently, eye size.
https://doi.org/10.1242/dev.167171
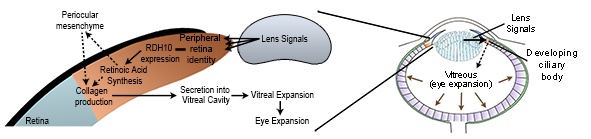
Figure 2. The lens controls eye size indirectly through controlling patterning of the peripheral optic cup and, consequently, production of the vitreous. Vitreous accumulation drives expansion of the eye in a similar fashion to air inflating a balloon.
Using RNA-seq on normal and lens-removed chicken eyes we have identified additional lens-regulated genes and signalling pathways. This includes genes with no current established role in eye development and genes associated with human eye disorders and disease. Using chicken, mouse and zebrafish embryo and human eye organoids, we are using gene expression and functional analyses to determine the importance of these genes for normal eye development and later risk of eye disease.
GUIDANCE OF RETINAL AXONS TO FORM FUNCTIONAL CONNECTIONS WITH BRAIN TARGET REGIONS
Visual information is transmitted to the brain via the axons of retinal ganglion cells. These connections are formed through the growth of retinal ganglion cell axons along a stereotypical pathway from the eye to higher visual processing centres. Failure of these connections to form normally or damage to the optic nerve due to traumatic injury or common eye disorders such as glaucoma, can result in severe visual impairment or blindness. Using expression analysis, in vitro assays of axon growth and guidance and mice genetics, my work has identified a number of molecules important for the growth and guidance of retinal ganglion cells axons from the eye to brain target regions. This includes:
(i) VEGF-A (vascular endothelial growth factor-A) and its receptor Neuropilin 1 (NRP1).
In collaboration with Professor Christiana’s group we demonstrated that VEGF-A acts directly on NRP1-positive retinal ganglion cells to provide growth promoting and attractive signals important for normal development of the binocular visual pathways.
https://doi.org/10.1016/j.neuron.2011.02.052
https://doi.org/10.1242/dev.115998

Figure 3. In absence of NRP1, which is expressed normally by retinal ganglion cell axons, midline crossing at the optic chiasm is impaired. Using a growth cone turning assay we demonstrated that VEGF-A, which is localised to the chiasm midline, is a potent chemoattractant for retinal ganglion cell axons.
Through their role in patterning blood vessel development, VEGF-A and NRP1 also impact indirectly on axon organisation in the developing optic pathway through regulating blood vessel formation.
https://doi.org/10.1242/dev.151621
(ii) DSCAM (Down’s Syndrome Cell Adhesion Molecule)
In collaboration with Dr Peter Fuerst’s group we demonstrated that DSCAM promotes fasciculation and growth of retinal ganglion cell axons, controlling the timing of when axons reach visual targets.
https://doi.org/10.1073/pnas.1618606114
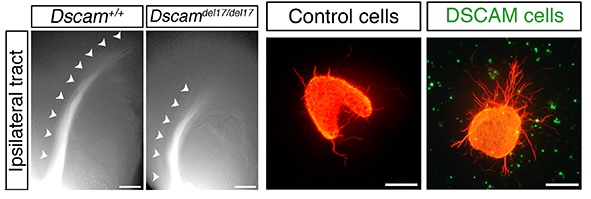
Figure 4. In mouse embryos lacking Dscam, which is expressed normally both by retinal ganglion cell axons and along the optic pathway, retinal ganglion cell axon extension along the optic pathway is impaired. In vitro DSCAM promotes retinal ganglion cell axon outgrowth.
We also have identified an essential role for DSCAM in regulating cell organisation in the retina important for visual function.
https://doi.org/10.1016/j.ydbio.2011.10.028
https://doi.org/10.1016/j.neuron.2009.09.027
(iii) Roundabout (Robo) receptors and their Slit ligands
We identified an essential role for inhibitory Slit-Robo signalling in serving a barrier function important for channelling retinal ganglion cell axon growth along their correct path as they extend through the retina and along the optic pathway. In the absence of Slit-Robo signalling retinal axons stray away from their correct path and fail to reach visual target regions.
https://doi.org/10.1016/S0896-6273(01)00586-4
https://doi.org/10.1523/JNEUROSCI.1342-06.2006
https://doi.org/10.1016/j.ydbio.2006.06.017
https://doi.org/10.1016/j.ydbio.2009.09.034
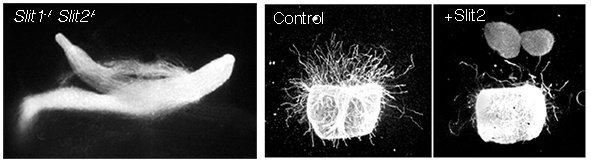
Figure 5. In mice lacking Slit1, Slit2, which are normally expressed surround the developing optic pathway, retinal axons can cross the midline in aberrant locations, extend in increased numbers into the contralateral optic nerve and stray into ectopic regions of the brain. In vitro Slits are potent inhibitors of retinal ganglion cell axon outgrowth.
(iv) EphrinB2 and its receptor EPHB1
We demonstrated an essential role for inhibitory EphrinB2/EphB1 signalling in patterning the binocular visual pathways.
https://doi.org/10.1016/J.NEURON.2003.08.017
Ongoing work is aimed at further elucidating the cellular and molecular mechanisms controlling the formation of functional connections between the eye and the brain.
Knowledge Exchange
Recent Public engagement activities:
2020 Fight For Sight Campaign to increase funding for visual impairment/blindness. Signed open letter to Guardian, interviewed by STV, article in Evening Express; Live Interview by BBC Radio Scotland
2018 Pint of Science (Portsmouth)
2013 Bring Your Own Brain III – Brain Injury and Repair
2010 onwards Supervision of work experience students from local High Schools gaining experience of working in a lab environment.
2005 Onwards Provided project placement for high school students undertaking a summer research project through the Nuffield Bursary Scheme. To date I have supervised 8 students under this scheme.
Collaborations
Professor Neil Vargesson, University of Aberdeen https://www.abdn.ac.uk/ims/research/profiles/n.vargesson
Professor Martin Collinson, University of Aberdeen https://www.abdn.ac.uk/ims/research/profiles/m.collinson
Professor Christiana Ruhrberg, UCL’s Institute of Ophthalmology https://www.ucl.ac.uk/ioo/research/ruhrberg-lab
Dr Peter Fuerst https://www.uidaho.edu/sci/biology/people/faculty/fuerst
Dr Eloisa Herrera Instituto de Neurociencias de Alicante CSIC-UMH, Alicante, Spain
https://eloisahgm.wixsite.com/herreralab/lab-members-c1h14
Dr Veronica Murcia-Belmonte Instituto de Neurociencias de Alicante CSIC-UMH, Alicante, Spain
Supervision
My current supervision areas are: Biomedical Sciences.
Supervision of PhD students:
Principal supervisor:
01/06/18 - Miss Viet Hang Le
01/10/15 – 30/09/19 Mr Jonathan Smith
01/10/13 – 30/04/18 Miss Samantha Brown
01/10/10 – 30/09/14 Miss Heather Walker
01/10/07 - 30/09/11 Miss Freyja Bruce
01/04/04 – 30/08/07 Miss Hannah Thompson - Research Assistant/Part-Time PhD Student
01/10/03 – 30/09/07 Mr Bennett Alakakone
Co-supervisor:
01/10/17 – Miss Amanda Berg (co-supervisor with Professor Neil Vargesson)
01/10/17 – Miss Sadia Shamsi (co-supervisor with Professor Neil Vargesson)
01/10/12 – 30/09/16 Miss Alexandra Diamond (co-supervisor with Dr Neil Vargesson)
01/10/11 – 30/09/14 Mr Domenico Alessio Panzica (co-supervisor with Professor Martin Collinson; Funded by The Anatomical Society)
01/10/09 – 30/09/13 Mr Christopher Mahoney (co-supervisor with Dr Neil Vargesson)
01/10/09 – 30/09/12 Mr Scott McMenemey (co-supervisor with Dr Neil Vargesson)
01/10/07 - 30/09/10 Miss Elizabeth Kilby (co-supervisor with Dr Neil Vargesson)
Supervision of MRes students:
01/10/14 – 30/09/15 Mr Jonathan Smith
01/10/12 – 30/09/13 Mr Lukasz Ciszewski
01/06/13 – 20/09/13 Mr Leandro Pucci
Training, Supervision and Mentoring of laboratory research staff:
01/01/18 – 31/12/18 Dr Verónica Murcia-Belmonte - Postdoctoral Research Fellow
01/04/12 – 30/03/16 Dr Freyja Bruce – Postdoctoral Research Fellow
01/02/09 - 31/01/12 Dr Susan Reijntjes - Postdoctoral Research Fellow
01/04/04 – 30/08/07 Miss Hannah Thompson - Research Assistant/Part-Time PhD Student
01/12/03 – 31/12/04 Dr Olivier Camand - Postdoctoral Research Fellow
01/04/02 – 31/01/06 Dr Subathra Poopalasundra - Postdoctoral Research Fellow (co-supervisor with Drs Alison Hardcastle and Mike Cheetham).
01/03/02 – 31/08/03 Dr Lisa Fagg - Postdoctoral Research Fellow
Funding and Grants
2020-2021 NHS Grampian Endowment Research Grant Investigation of the role of myopia susceptibility genes in early eye development. £11K (PI; co-applicants Prof Neil Vargesson, Prof Martin Collinson).
2017-2018 Wellcome Trust Multi-User Equipment Grant. Zeiss LSM880 confocal microscope with Airyscan detector for super-resolution imaging at the University of Aberdeen. £333K (PI; co-applicants Professors Alistair Brown, Gordon Brown, Cosimo De Bari, Anne Donaldson, Neil Gow, Stefania Spano and Dr Nicola Mutch).
2016-2018 Generalitat Valenciana (Conselleria d'educació, investigació, cultura i esport) The role of Zic2 in the retinal marginal zone and eye growth – implications in myopia and hyperopia. €93K (Fellowship to Veronica Murcia-Belmonte; co-Mentor with Dr Eloisa, Herrera, Alicante).
2015-2017 The University of Aberdeen Development Trust Research into corneal blindness. £25K (PI Prof Martin Collinson; co-applicant with Prof Neil Vargesson)
2012-2015 BBSRC Project Grant Understanding the function and signalling mechanisms of VEGF-A and VEGF-C in optic chiasm development. £580K (PI; joint application with Professor Christiana Ruhrberg, UCL).
2012-213 RS Macdonald Charitable Trust Equipment for nerve growth cone turning assays. £22K (PI)
2009-2012 Wellcome Trust Project Grant Defining the role of vascular endothelial growth factor (VEGF-A) in commissural axon guidance at the optic chiasm. £342K (PI; joint application with Professor Christiana Ruhrberg, UCL).
2004-2006 Wellcome Trust Project Grant An investigation of the role of Robo-Slit interactions in regulating mammalian visual system development. £119K (PI)
2002-2005 Wellcome Trust Project Grant Functional analysis of Nyctalopin: probing the development of retinal architecture. (PI Prof Alison Hardcastle UCL; Co-applicant with Prof Mike Cheetham).
2002-2004 Wellcome Trust Project Grant The role of Slits and Semaphorins in regulating retinal ganglion cell axon guidance. £176K (PI)
- Teaching
-
Teaching Responsibilities
Course Co-ordiantor AN3301 (Human Embryonic Development) and MB5524 (Applied Developmental Biology)
Non-course Teaching Responsibilities
Degree Programme Co-ordinator MSc in Reproductive and Developmental Biology.
- Publications
-
Page 3 of 3 Results 51 to 53 of 53
Electric field-directed growth and branching of cultured frog nerves: effects of aminoglycosides and polycations
Journal of Neurobiology, vol. 26, no. 4, pp. 523-536Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1002/neu.480260406
Growing Nerves in an Electric Field
NeuroProtocols, vol. 4, pp. 134-141Contributions to Journals: Literature ReviewsNeurotransmitters, second messengers and protein kinase C may underlie orientation of cultured frog nerves in an applied electric field
Journal of Physiology - Paris, vol. 86Contributions to Journals: Articles- [ONLINE] View publication in Scopus
