
PhD CPsychol AFBPsS
Personal Chair
- About
-
- Email Address
- s.maclennan@abdn.ac.uk
- Telephone Number
- +44 (0)1224 438125
- Office Address
Academic Urology Unit Health Sciences Building (2nd Floor) University of Aberdeen Foresterhill Aberdeen AB25 2ZD
- School/Department
- School of Medicine, Medical Sciences and Nutrition
Biography
I am a psychosocial oncologist and a health psychologist with expertise in qualitative research methodology. I pursue an interdisciplinary approach building multi-stakeholder working in cancer care and have established global partnerships of academics, patients, non-profit organisations, professional organisations and policy makers.
Aberdeen Applied Cancer Research Group (ICANTREAT):
I lead the Aberdeen Applied Cancer Research Group (ICANTREAT)
The group's research strategy focuses on understanding the impact of cancer and treatment on individual’s lives and methods for involving different stakeholder groups, particularly patients, in the design and delivery of care.
I am Deputy Director of the Academic Urology Unit, Director of Operations for UCAN (Urological CANcer charity) and hold an honorary contract with NHS Grampian. I am an Honorary Research Fellow, Birkbeck University of London. I am a Chartered Psychologist and Associate Fellow of the British Psychological Society, and a registered health psychologist (Health & Care Professions Council).
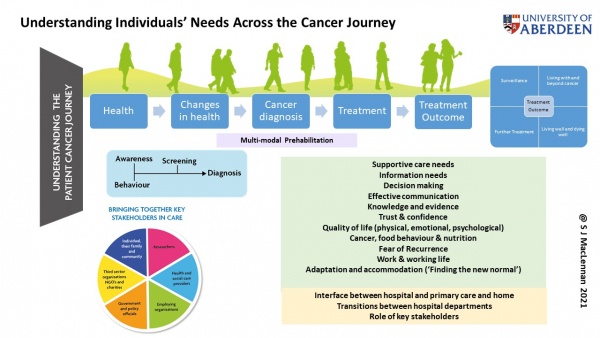
Current projects include:
Stakeholder engagement in the design and implementation of cancer care:
ICANBE – Managing Fear of Cancer Recurrence
EVOLVE – Giving cancer patients a meaningful voice within the design and delivery of clinical practice guidelines
ICANTREATNEPAL / ICANTREATINDIA – Increasing participation in cancer screening and treatment in India & Nepal (International Global Health Network)PARTNER –Men’s prostate cancer treatment preferences
OPTIMA – Optimal treatments for patients with solid tumours in Europe through AIPIONEER – Enhancing prostate cancer diagnosis and treatment through the power of big data in Europe
Example of Impact:

I am to keen to supervise PhD students interested in working in cancer survivorship and supportive care.
Memberships and Affiliations
- Internal Memberships
-
University Committees:
Chair - Institute of Applied Health Sciences (IAHS) Research Strategy Group
Chair - Institute of Applied Health Sciences (IAHS) Health, Safety & Well-being Committee - External Memberships
-
Current External Membership:
Member - European Cancer Organisation Steering Committee for Survivorship and Quality of Life (2021 onwards)
Member - European Cancer Organisation Survivorship Focused Topic Network (2021 onwards)
Member - European Cancer Organisation Workforce Focused Topic Network (2021 onwards)
Member – Executive Committee, British Psychosocial Oncology Society (2016 - onwards)
Member – National Cancer Research Institute –Living With and Beyond Cancer Late Effects Workstream (2019 onwards)
Member – Patient Advocacy Group, European Association of Urologists (2018 - onwards)
Member – Breast Cancer Now, Grants Award Committee (2018 – onwards)
Member – ESRC Peer Review College (2017 – onwards)Previous External Membership:
Member – MRC Strategic Skills Panel (2013 - 2017)
Member – Grants Award Panel, Carnegie Trust
Member – Executive Committee, Division of Health Psychology Scotland (2016 - 2017)
External Examiner (MSc) MSc Health Psychology, University of Westminster (2008 - 2011)
Member - BPS working party on ‘Work and Health’
Elected Member – Training Committee, Division of Health Psychology (2006 - 2008)
Elected Member - Executive Committee, Division of Health Psychology (2006 – 2008)
Assessor - Board of Assessors, Division of Health Psychology (2002 - 2008)
Examiner – Paper 4 (Health Psychology), Qualifying Examination (2002 – 2012)
Member - BPS working party on ‘Psychological Debriefing’(2001 - 2002)
Latest Publications
CATHETER II: a randomised controlled trial comparing the clinical and cost effectiveness of various washout policies versus no washout policy in preventing catheter associated complications in adults living with long term catheters
BMJ Open, vol. 14, no. 12, e087203Contributions to Journals: ArticlesWeb-based interventions for fear of cancer recurrence: a scoping review with a focus on suggestions for the development and evaluation of future interventions
PloS ONE, vol. 19, no. 11, e0312769Contributions to Journals: ArticlesPatient and health care professionals’ perception of weekly prophylactic catheter washout in adults living with long-term catheters: Qualitative Study of the CATHETER II Trial
RCOG World Congress 2024Contributions to Conferences: PostersPatient and health care professionals’ perception of weekly prophylactic catheter washout in adults living with long-term catheters: Qualitative Study of the CATHETER II Trial
The International Continence SocietyContributions to Conferences: PostersRCT comparing the clinical and cost effectiveness of various catheter washout policies
RCOG World Congress 2024Contributions to Conferences: Posters
- Research
-
Research Overview
I am a psychosocial oncologist and a health psychologist with expertise in qualitative research methodology. I pursue an interdisciplinary approach building multi-stakeholder working in cancer care and have established global partnerships of academics, patients, non-profit organisations, professional organisations and policy makers. Key to this is an understanding of processes within the delivery of routine clinical care. My research expertise includes process evaluation.
My research strategy focuses on understanding the impact of cancer and treatment on individual’s lives and methods for involving different stakeholder groups, particularly patients, in the design and delivery of care.
Research Areas
Applied Health Sciences
Nutrition and Health
Psychology
Research Specialisms
- Health and Social Care
- Applied Psychology
- Health Psychology
- Oncology
Our research specialisms are based on the Higher Education Classification of Subjects (HECoS) which is HESA open data, published under the Creative Commons Attribution 4.0 International licence.
Current Research
I currently have eleven funded projects (total awards £5,644,624) across three themes:
Stakeholder engagement in the design and implementation of cancer care (Developed and Lead Projects):
- ICANBE – Fear of Cancer Recurrence
- EVOLVE – Giving cancer patient’s a meaningful voice within the design and delivery of clinical practice guidelines across Europe
- PIONEER – Enhancing prostate cancer diagnosis and treatment through the power of big data in Europe; €12,000,000 EU grant (Lead for Aberdeen)
- ICANTREAT – Increasing participation in breast cancer screening and treatment in Nepal and in Uganda; screening and treatment inequalities in India (International Global Health Research Group)
The design and implementation of better cancer care (Co-applicant):
- OPTIMA – Optimal treatments for patients with solid tumours in Europe through AI
- PARTNER – Men’s prostate cancer treatment preferences
- ICAN – Improving understanding of Urological cancer (Cancer Research Group)
- RCOS – Kidney Cancer core outcome set
The design and delivery of care and after care - Urology and other long-term conditions (Co-applicant)
- SIMS LTFU – Surgical management of female stress urinary incontinence
- CATHETER II – Understanding the patient experience of care; embedded qualitative study within a clinical trial to look at Catheter washout policies
- PACFIND – Patient-centred care for Fibromyalgia
Funding and Grants
£1,185,670 Arthritis UK (2019 – 2024). Patient-centred Care for Fibromyalgia: New pathway Design (PACFIND). MacFarlane, G [PI], Maclennan SJ et al. [co-applicants]
£24,537 NHS Grampian. (2018-2019). Achieving Self-Directed Integrated Cancer Aftercare (ASICA): developing digitally supported cancer aftercare to achieve high quality, equitable care for diverse populations. Murchie, P [PI], Maclennan SJ et al. [co-applicants]
£9,805 GCRF-IPPF3 (2018). Increasing participation in breast cancer screening and treatment within Manipal, India (CANTREAT). MacLennan, SJ., & Poobalan, A.
£5,341,011 EU IMI (2018-2023). Prostate Cancer Diagnosis and TreatmeNt Enhancement through the Power of Big Data in EuRope – PIONEER (2018 – 2023). PIONEER Consortium – MacLennan SJ – lead applicant for University of Aberdeen group.
£2,271,424 NIHR HTA (2018-2023) Randomised Controlled Trial CompAring THE Clinical And CosT- Effectiveness Of Various Washout Policies Versus No Washout Policy In Preventing Catheter Associated Complications In Adults Living With Long-Term Catheters (CATHETER II Study). Abdel-Fattah, M. [PI], Maclennan SJ et al. [co-applicants]
£175,000 NHS Grampian Endowments (via UCAN) (2018-2021). EVOLVE: Giving Patients a Meaningful Voice in the Design and Delivery of Care. S J MacLennan
£50,000 European Association of Urology (2017-2018). Review of Renal Cell Carcinoma: Muscle-invasive & Metastatic Bladder Cancer (Lymph node Dissection) & Penile Cancer. N’Dow, J., & MacLennan, S.J.
£123,357 UCAN (2017- 2019). Meeting the Needs of Patients: Developing Core outcome Sets for Urological Cancer. MacLennan, S.J.
€1,070 EU COST action STSM-IS1211-35195 (2016). Cancer and Work Participation: Research visit to Dr Angela de Boer, Coronel Institute for Occupational Health, Academic Medical Centre, Amsterdam. MacLennan, SJ.
£11,548 NHS Grampian Endowments Award (2015 – 2016). Understanding the hard choices made by working women with breast cancer between treatment compliance and working on: clinical vs economic survival. Improving the Role of the NHS in Grampian. MacLennan SJ., Cox T., N’Dow, J & Heys, S.
£750 Santander Mobility Award (2015), University of Aberdeen. Developing the METIS Collaboration: Cancer Survivorship, Work & Working Life. MacLennan SJ.
£29,500 ESRC (2014 – 2017) Social Science Perspectives on the Working Lives of Those with Cancer: Psychosocial, Organisational and Economic Perspectives. Cox, T., MacLennan, SJ., Hassard, J., & Brown, H.
£25,000 Birkbeck University of London (2014) The Metis Collaboration: Cancer Survivorship, Work & Working Life. MacLennan, SJ.
£109,646 Macmillan Cancer Support (2013 – 2015) Cancer Survivorship: The Patient Journey, Work & Working Life. MacLennan, SJ., Cox, T., & N’Dow, J.
£1,426,755 Health Technology Assessment Programme (HTA) (2013 – 2017)Therapeutic Interventions for Stones of the Ureter (TISU): a multicentre randomised controlled trial of extracorporeal shockwave lithotripsy, as first treatment option, compared with direct progression to ureteroscopic retrieval, for ureteric stones. (McClinton,S., N’Dow, J., MacLennan, GS., Kilonzo, M., Keeley, F., Anson, K., Clark, C., Pickard, R., Norrie, J., MacLennan, SJ., Thomas, R., Starr, K., Burgess, N., Lam, T., Kurban, L.)
£292,253 Health Technology Assessment Programme (HTA) (2012 – 2015) Ablative therapy for men with localised prostate cancer (Ramsay, C., Pickard, R., Vale, L., Lam, T., Mowatt, G., MacLennan, SJ., Rushton, S., N’Dow, J., Merseburger, A., Shirley, M., & Heidenreich, A. )
£176,903 Cancer Research Aberdeen & North East Scotland (CRANES) (2012 – 2015) Development of core outcomes for surgical management of localised prostate cancer to support decision-making by patients, clinicians and policy makers (Lam, T., N’Dow, J., MacLennan, SJ., Ramsay, C., & Campbell, M)
£24,680 The Prostate Cancer Charity Scotland (2011 – 2012) Support groups for men who have prostate cancer, their families and friends: identifying best practice models (MacLennan, SJ., Skea, Z, N’Dow, J & McCann, S)
£14,042 Scottish Cancer Research Network (2011 – 2012) Information for choice in urological cancer: What people need, prefer and use (MacLennan, SJ., & Skea, Z)
£10,317 Scottish Cancer Research Network (2010 – 2011) Delivering Peer Support Interventions in Urological Cancer (Employing a research nurse) (MacLennan, SJ., & N’Dow, J)
£150,700UCAN (Urological Cancer Charity) (2008 – 2013) Addressing the gaps in evidence for treatment of urological cancers (Employing a systematic reviewer) (N’Dow, J., MacLennan, SJ., & Imamura, M)
£165,975 The Scottish Government – CSO (2009 – 2012) Postdoctoral Training Fellowship in Health Services and Health of Public Research – The Acceptability and Usefulness of a Trial Participation Decision Aid: A Mixed Methods Study of Patients and Clinicians in the UK (Schumm, K., Campbell, M., Ramsay, C., N’Dow, J., Skea, Z., & MacLennan, SJ)
£245,482 UCAN (Urological Cancer Charity) (2009 – 2013) Information needs of those living with urological cancer (MacLennan, SJ)
£147,000 Macmillan in partnership with UCAN (2009 – 2012) Making life better for those living with urological cancer (MacLennan, SJ)
Datasets
-
Health status and associated factors of middle-aged and older adult cancer survivors in India: results from the Longitudinal Ageing Study in India
Abstract Background The number of persons who have survived cancer has been increasing in India as elsewhere due to advances in detection and treatment of this disease. However, evidence on the standardised number of cancer survivors, their character...- DOI
- 10.6084/m9.figshare.c.6262030
- Publisher
- Figshare
-
Data from: Health status and associated factors of middle-aged and older adult cancer survivors in India: results from the Longitudinal Ageing Study in India
Abstract Background The number of persons who have survived cancer has been increasing in India as elsewhere due to advances in detection and treatment of this disease. However, evidence on the standardised number of cancer survivors, their character...- DOI
- 10.6084/m9.figshare.c.6262030.v1
- Publisher
- Figshare
-
Data From Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review
- DOI
- 10.1186/s43058-023-00498-0
- Publisher
- University of Aberdeen
-
Data From Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review
- DOI
- 10.1186/s43058-023-00498-0
- Publisher
- University of Aberdeen
-
Data From Influences on androgen deprivation therapy prescribing before surgery in high-risk prostate cancer
- DOI
- 10.1002/bco2.411
- Publisher
- University of Aberdeen
-
Data From Influences on androgen deprivation therapy prescribing before surgery in high-risk prostate cancer
- DOI
- 10.1002/bco2.411
- Publisher
- University of Aberdeen
- Teaching
-
- Publications
-
Page 3 of 5 Results 51 to 75 of 124
EVOLVE: a framework for meaningful patient involvement in clinical practice guideline development and implementation
Cochrane Database of Systematic Reviews, vol. 9 Suppl 1, pp. 26Contributions to Journals: AbstractsNo turning back’ Psycho-oncology in the time of COVID-19: Insights from a survey of UK professionals
Psycho-Oncology, vol. 29, no. 9, pp. 1430-1435Contributions to Journals: ArticlesAuthor Correction: Introducing PIONEER: a project to harness big data in prostate cancer research (Nature Reviews Urology, (2020), 17, 6, (351-362), 10.1038/s41585-020-0324-x)
Nature Reviews Urology, vol. 17, pp. 482Contributions to Journals: Comments and Debates- [ONLINE] DOI: https://doi.org/10.1038/s41585-020-0355-3
- [ONLINE] View publication in Scopus
- [ONLINE] Springer Nature SharedIt link to author correction.
Benefits of an embedded qualitative study within clinical trials: Patient values and preferences
35th Annual EAU CongressContributions to Conferences: AbstractsIntroducing PIONEER: a project to harness big data in prostate cancer research
Nature reviews. Urology, vol. 17, pp. 351-362Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1038/s41585-020-0355-3
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/15415/1/Omar_NRU_AAM_In_principle.pdf
- [ONLINE] View publication in Scopus
. PIONEER’s systematic review of outcomes reported in effectiveness trials for interventions in locally advanced prostate cancer
11th European Multidisciplinary Congress on Urological CancersContributions to Conferences: AbstractsPIONEER’s update and integration of a localised prostate cancer core outcome set for effectiveness trials and a standard set for clinical practice
11th European Multidisciplinary Congress on Urological CancersContributions to Conferences: AbstractsEVOLVE: Designing a model of meaningful patient involvement in guideline development
European Urology Supplements, vol. 18, no. 1, pp. e2117Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/s1569-9056(19)31534-9
TISU: Extracorporeal shockwave lithotripsy, as first treatment option, compared with direct progression to ureteroscopic treatment, for ureteric stones: study protocol for a randomised controlled trial
Trials, vol. 19, no. 1, pp. 1-11Contributions to Journals: ArticlesCare pathways for the management of localised and locally advanced prostate cancer:: Experience of the EAU guidelines office
33rd Annual EAU Congress 2018, pp. PT075Contributions to Conferences: PostersCare pathways for the management of metastatic and castration-resistant prostate cancer in the era of novel therapeutic options:: Experience of the EAU guidelines office
33rd Annual EAU Congress 2018Contributions to Conferences: Posters“Throughout the cancer patient's journey, there ought to be a discussion about work”: The role of GPs in Scotland
Psycho-Oncology, vol. 27, no. 1, pp. 343-346Contributions to Journals: ArticlesThe relationship between social support and health-related quality of life in patients with antiphospholipid (hughes) syndrome
Modern Rheumatology , vol. 28, no. 1, pp. 147-155Contributions to Journals: ArticlesA core outcome set for localised prostate cancer effectiveness trials
BJU International, vol. 120, no. 5B, pp. E64-E79Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1111/bju.13854
- [ONLINE] View publication in Scopus
A core outcome set for localised prostate cancer effectiveness trials
BJU International, vol. 120, no. 5B, pp. E64-79Contributions to Journals: ArticlesChanging current practice in urological cancer care: Providing better information, advice and related support on work engagement
European Journal of Cancer Care, vol. 26, no. 5, e12756Contributions to Journals: ArticlesChanging Current Practice in Urology: Improving Guideline Development and Implementation Through Stakeholder Engagement
European Urology, vol. 72, no. 2, pp. 161-163Contributions to Journals: Editorials- [ONLINE] DOI: https://doi.org/10.1016/j.eururo.2017.02.008
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/10077/1/EURUROL_S_17_00216.pdf
The Relationship Between Social Support and Health-Related Quality of Life in Patients with Anti-Phospholipid Syndrome
Annual Meeting of the British-Society-for-Rheumatology, British-Health-Professionals-in-Rheumatology and the British-Society-for-Paediatric-and-Adolescent-Rheumatology (Rheumatology), pp. 110-110Contributions to Journals: AbstractsPatient-centered Care in Maternity Services: A Critical Appraisal and Synthesis of the Literature
Women's Health Issues, vol. 26, no. 1, pp. 100-109Contributions to Journals: ArticlesExploring the Role of General Practitioners in the Provision of Work-Related Advice, Information and Support to People with Cancer in Scotland: A Grounded Theory Study
NHS Scotland Research Conference 2015Contributions to Conferences: PostersSurgical management for localised penile cancer: [Cochrane Protocol]
Contributions to Journals: Registered ReportsAblative therapy for people with localised prostate cancer: a systematic review and economic evaluation
Health Technology Assessment, vol. 19, no. 49, pp. 1-524Contributions to Journals: ArticlesThe influences of nursing transformational leadership style on the quality of nurses’ working lives in Taiwan: a cross-sectional quantitative study
BMC Nursing, vol. 14, 33Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1186/s12912-015-0082-x
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/4631/1/s12912_015_0082_x.pdf
Which type of indwelling urethral catheters should be used for short-term catheterisation in hospitalised adults?: Cochrane Systematic Review of the evidence
21rst Annual Scientific Meeting of the UK Continence SocietyContributions to Conferences: AbstractsSurgical management for localised penile cancer
Cochrane Database of Systematic Reviews, vol. 2015, no. 3, CD011533Contributions to Journals: Articles- [ONLINE] https://doi.org//10.1002/14651858.CD011533
- [ONLINE] DOI: https://doi.org/10.1002/14651858.CD011533

