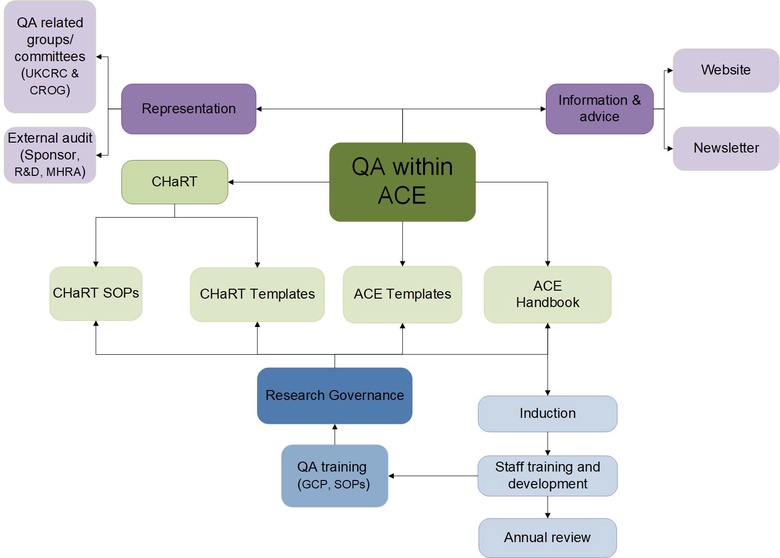
Please click on the boxes below to find out more information about QA in ACE.
- Annual Review
-
The Centre follows the Staff Annual Review process which aims to help all members of staff develop their full potential, enhance their sense of personal fulfilment at work and their ability to take advantage of opportunities to develop their career. Further details are available here.
As part of this, an annual review meeting is carried out for all ACE staff. Meetings take place on a one-to-one basis between an employee and their line manager or other nominated staff member.
- CHaRT Standard Operating Procedure (SOPs)
-
All CHaRT trials are governed by the CHaRT Standard Operating Procedure (SOP) book developed for managing trials to a high standard and thus meeting these ethical, regulatory and governance standards. A copy of the SOP book can be found here.
- CHaRT and ACE Templates
-
General CHaRT and ACE document templates (e.g. headed paper, presentation slides, poster presentations etc.) are available in the resources repository on Q-Pulse.
This resource can be accessed by ACE staff only.
- External Audit
-
ACE is subject to audit and inspection by external independent organisations, competent authorities and local University and NHS R&D groups. The audits, monitoring visits and inspections that ACE are likely to be involved with are carried out by:
- Sponsor (local or external)
- R&D (NHS Grampian)
- MHRA (Medicines and Healthcare Products Regulatory Agency)
In order to prepare for such visits, the QA manager will act as a point of contact and is responsible for liaising with the appropriate staff, assisting with the preparation of any documentation required, responding to requests during the visit, coordinating the response to any requirements identified in the auditor's report etc.
In addition, it is imperative that all ACE staff are well versed with the appropriate ACE policies and procedures (staff handbook, protecting information policy, CHaRT SOP book etc), and that they also take responsibility for documenting and maintaining a personal training record.
- ACE Handbook
-
The ACE handbook has been designed to bring together in one source all the commonly used 'core information' needed by staff and post-graduate (PGR) students to familiarise themselves with the Aberdeen Centre for Evaluation (ACE) during their first few days. It also serves as a reference document, to remind staff and PGR students of procedures, policies etc., during their time with ACE.
A copy of the handbook is available on Q-Pulse.
- Induction
-
In order to help staff settle in quickly and successfully to their new job in the Aberdeen Centre for Evaluation (ACE), new staff will be assigned a 'buddy' who will take them through an induction checklist. This checklist lists some of the practical things you need to know and do, at a departmental level.
The Protecting Information Policy (PIP) form sets out how data must be handled to ensure compliance with the Data Protection Act 1998. All members of staff are made aware of this policy during their induction session and signed forms are retained by the Quality Assurance manager. A copy of the Policy is available on Q-Pulse.
The induction 'buddy' forms a link between the new starter and ACE in which they will be working. Their main role is to help new members of staff with their orientation into working practices in ACE and, to familiarise them with their immediate surroundings as well as a tour of the campus.
Copies of both induction and buddy checklists can be found on Q-Pulse.
In addition to the ACE induction, all staff are invited to attend one of the University's monthly induction events. Please see the University website for further details.
- Newsletter
-
The QA newsletter provides information on QA in ACE, as well as relevant training courses (e.g. GCP, appraisals etc.) and regulatory updates. Please find the current issue here.
Current Issue
Past copies of the newsletter are available in PDF format upon request.
- QA Related Groups / Committees
- QA Training
-
See CHaRT SOPs
- Research Governance
-
Research Governance can be defined as the broad range of regulations, principles and standards of good practice that exist to achieve, and continuously improve, research quality across all aspects of healthcare in the UK and worldwide. The Research Governance Framework is a document that sets out a framework for the governance of research in health and community care.
The University of Aberdeen and NHS Grampian also have a website that provides information on Clinical Research Governance and Quality Assurance.
Other websites which may be of interest are:
- Integrated Research Application System (IRAS)
- National Research Ethics Service (NRES) - now part of the Health Research Authority (HRA)
- Honorary Contracts & Research Passports
- Staff Development and Training
-
The Centre follows the University of Aberdeen Staff Development Policy; full details are available here. The University runs a number of training and staff development courses to support all staff who wish to continue their personal and/or professional development. For further information please click here.
Applications to attend any conferences or training courses should be approved by the appropriate Programme Director or the Director. All staff must complete an online Conference & Training Attendance Request Form.
All staff are expected to keep an up-to-date 'Staff Development Manual' or equivalent which will include their CV, annual review objectives, staff training courses attended.
In addition to maintaining a staff development manual (or equivalent), it is good practice for all staff to keep an up-to-date 'training log'. A training log is an ongoing, cumulative list of all internal and external training, attendance at conferences, workshops etc. For training where no certificate is provided, it is advisable to keep a copy of any handouts or presentation from that training course to verify attendance as well as information on the topics covered. A training log template can be found on Q-Pulse.
Flowchart showing QA in ACE