
FPhysiol
Senior Lecturer
- About
-
- Email Address
- g.s.bewick@abdn.ac.uk
- Telephone Number
- +44 (0)1224 437398
- Office Address
Rm 6:18
Institute of Medical Sciences
School of Medicine, Medical Sciences & Nutrition
Foresterhill
Aberdeen AB25 2ZD
Scotland
United Kingdom
- School/Department
- School of Medicine, Medical Sciences and Nutrition
Biography
Self-funding PhD applications invited
Self-funding PhD applications invited
Qualifications
- FPhysiol Physiology2020 - The Physiological Society
- PhD Neuroscience1986 - King's College London
- BSc (Hons) Zoology & Animal Physiology1979 - University of East Anglia
Memberships and Affiliations
- Internal Memberships
-
Lead Safety Co-ordinator, Institute of Medical Sciences, School of Medicine, Medical Sciences & Nutrition
Course Co-ordinator (Joint) SM3002 Frontiers of Biomedical Sciences
Personal Tutor
Aberdeen Cardiovascular and Diabetes Centre
Translational Neuroscience Programme
- External Memberships
-
Fellow of The Physiological Society of Great Britain and Ireland
Society for Neuroscience
British Neuroscience Association
Federation of European Neuroscience
British Society for Cell Biology
British Society for Developmental Biology
Scottish Neuroscience Group
Euan MacDonald Centre for MND Research
Latest Publications
Biophysical model of muscle spindle encoding
Experimental Physiology, vol. 109, no. 1, pp. 55-65Contributions to Journals: ArticlesThe atypical 'hippocampal' glutamate receptor coupled to phospholipase D that controls stretch-sensitivity in primary mechanosensory nerve endings is homomeric purely metabotropic GluK2
Experimental Physiology, vol. 109, no. 1, pp. 81-99Contributions to Journals: ArticlesStanniocalcin-2 inhibits skeletal muscle growth and is upregulated in functional overload-induced hypertrophy
Physiological reports, vol. 11, no. 15, e15793Contributions to Journals: ArticlesDevelopment of abnormalities at the neuromuscular junction in the SOD1-G93A mouse model of ALS: dysfunction then disruption of postsynaptic structure precede overt motor symptoms
Frontiers in Molecular Neuroscience, vol. 16, 1169075Contributions to Journals: ArticlesMolecular characterization of the intact mouse muscle spindle using a multi-omics approach
eLife, vol. 12, e81843.Contributions to Journals: Articles
Prizes and Awards
2016-2020 - Trustee, The Physiological Society
- Research
-
Research Overview
We study how nerve signal strength is kept in the 'goldilocks zone' - not too strong, not too weak, but just right. In whatever we do, signalling adapts. This happens in both motor and sensory nerve endings. We study the nerve-muscle system, due to its importance for movement to us as animals, plus it is accessible, has large nerve endings and it changes use throughout life.
Understanding Movement Detection (Sensory Feedback)

Muscle length detector nerve terminals, labeled with
FM1-43 - a transmitter vesicle label.
(Dr Anna Simon, University of Aberdeen)Here, our major focus is to find out why the nerve endings that detect movements of our limbs and blood vessels also secrete chemicals that act back to regulate their own sensitivity. With Dr Robert Banks (Durham University), we discovered this system of chemical secretion from synaptic-like vesicles. It seems important, as we showed that all such endings have this system. We are now trying to incorporate this into an integrated model of how these endings work, and so asking why they need such a control mechanism that can double the ending's sensitivity or even turn it off entirely!
Understanding Motor Nerve Function (Motor systems)
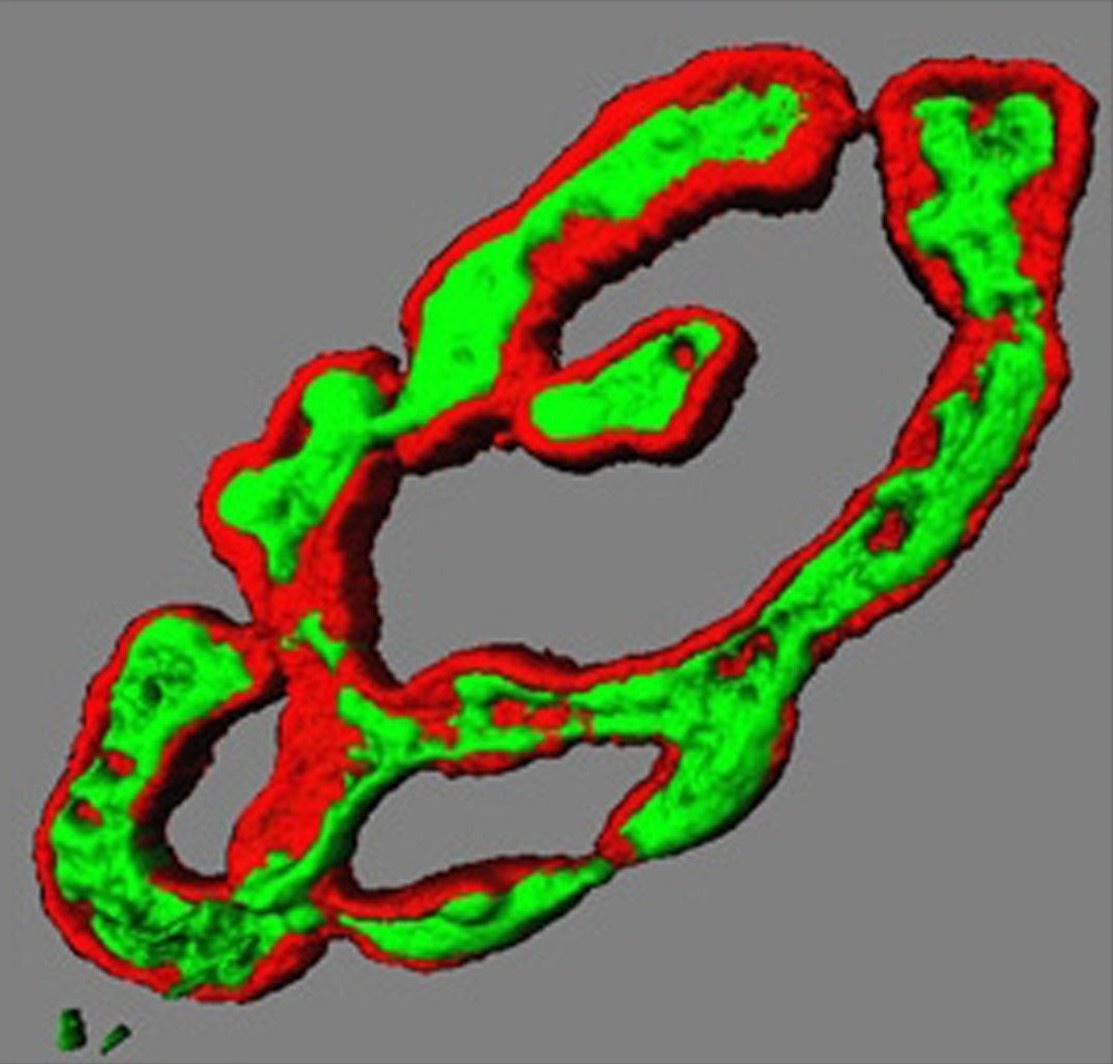
Transmitter vesicles (green) in motor nerve
terminal precisely overlie receptors on
muscle fibre surface (red). 3D render.
(Dr Brian Reid, University of Aberdeen)To move our muscles, an electrical signal triggers release of chemicals (neurotransmitter) from motor nerve endings on each muscle cell. These contacts form very early in development, yet the same nerve ending is present throughout life. We want to understand how the same nerve terminal knows how and when to change neurotransmitter release throughout life. It releases minute amounts in tiny baby muscles then enormous amounts in big adult muscles. Even further, they adapt to release much more in giant powerlifter muscle cells, or to maintain a constant release in muscles of distance runners. In all cases, neurotransmitter release adapts to be just enough to trigger muscle contraction in the size of the muscle cell it innervates, no matter how big or how often it is used. We want to understand the nerve-muscle signalling that adapts to meet these demands of growth, development and changes in in vivo activity.
Neurological disease treatments
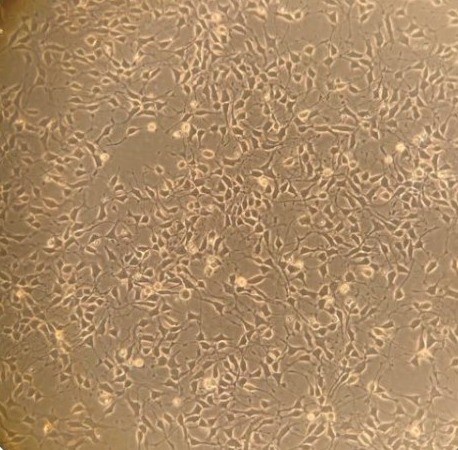
Human ALS - stem cell-derived motor
nerve cells in culture.
(Abigail Dos Santos, PhD student)These studies not only help us to understand how our wonderful nervous system works, but these natural regulatory systems are potential drug targets for treating symptoms in many diseases. Muscle weakness is often from poor neurotransmitter release or muscle response to it, e.g. myasthenia gravis, and congenital myasthenic syndromes. In motor neurone disease (aka MND or ALS), we think function in sick nerve terminals might be supported to extend the time signalling is useful, alleviating symptoms for at least a little longer. Conversely, disrupted motor control (ataxia) seems to reflects poor sensory terminal function. Similar types of endings around blood vessels stretch and relax, monitoring our blood pressure. We are exploring their potential as drug targets for high blood pressure, which is currently the world's biggest mortality risk factor.
Research Areas
Accepting PhDs
I am currently accepting PhDs in Biomedical Sciences.
Please get in touch if you would like to discuss your research ideas further.
Research Specialisms
- Neuroscience
- Physiology
Our research specialisms are based on the Higher Education Classification of Subjects (HECoS) which is HESA open data, published under the Creative Commons Attribution 4.0 International licence.
Current Research
Our two major lines of investigation are:
How do movement-detecting nerve endings work and adapt?
Our latest model of how these nerve endings work is this:
At rest, a chemical (glutamate) constantly dribbles from vesicles, keeping a receptor activated linked to PLD. This keeps the ending sensitive to movement. If blocked, the ending loses the ability to respond. Movement opens pores (channels) in the nerve terminal, letting positive sodium (Na+) ions in, making the inside positive. This opens different (voltage-sensitive) Na+ channels, producing a burst (afferent discharge) of electrical signals (action potentials) carried back to the central nervous system. These then open channels to allow calcium (Ca2+) in, then potassium (K+) out. K+ leaving moderates the afferent discharge frequency. Thus, the brain gets an urgent high-frequency signal when movement starts (meaning 'pay attention'), that slows to a modest level (meaning 'this is the movement happening'). Movement also lets Ca2+ into the terminal. This increases glutamate release, to maintain the terminal's ability to detect movement. We are investigating exactly how this glutamate system that we discovered regulates movement sensitivity. (Animation produced by Media Services and Medical Illustration, University of Aberdeen.© (2022) Guy Bewick, University of Aberdeen).

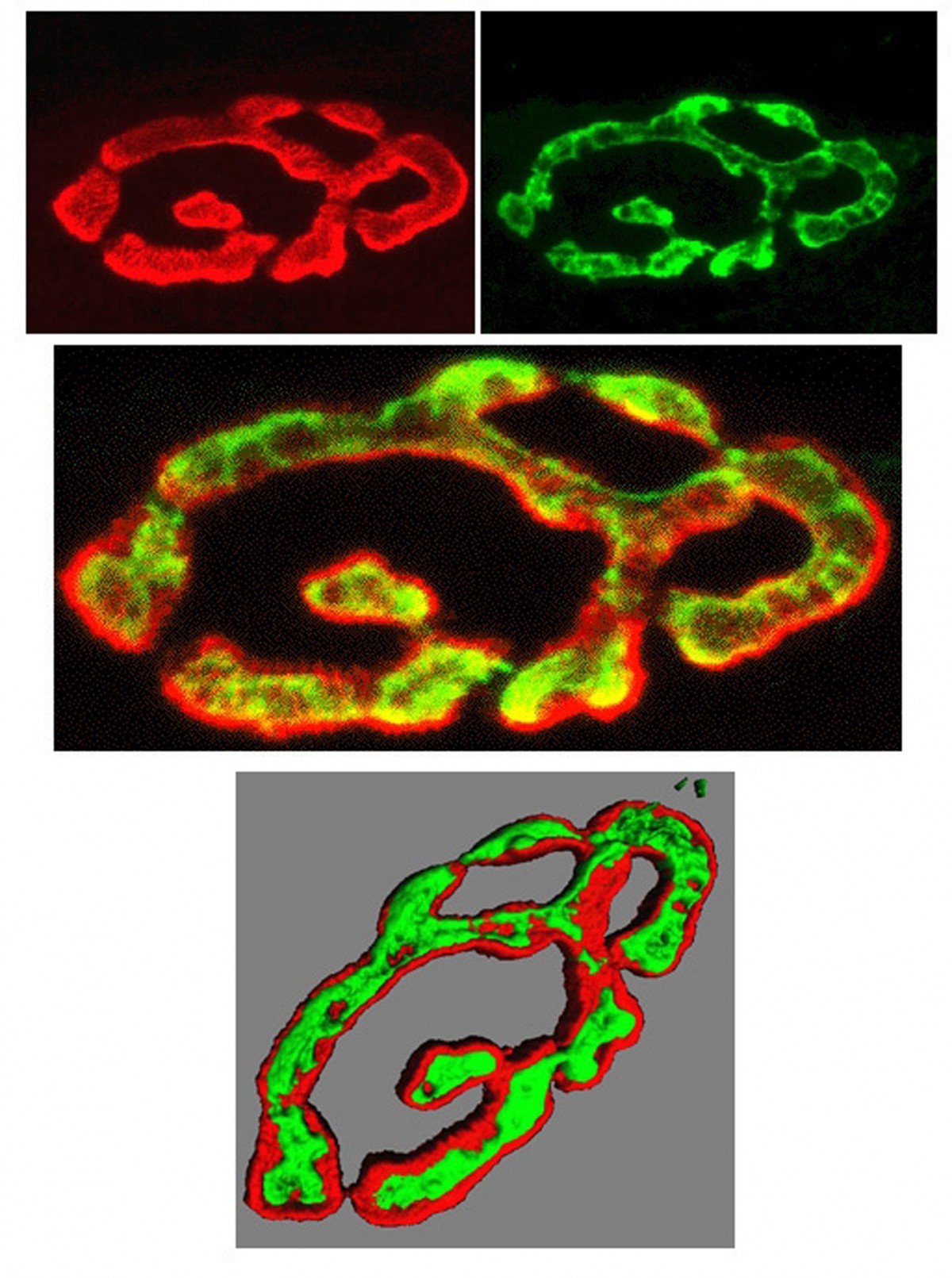
3 length detectors in a skeletal muscle.
(Gillian Milne, Microscopy Facility,
University of Aberdeen)Mechanosensory endings tell us where our arms and legs are, whether we're touching anything, and monitor our blood pressure. Tiny vesicles, just like those releasing neurotransmitter, occur in all such endings throughout the animal kingdom. Their ubiquity suggests they are probably very important and we have shown that indeed they are. In muscle stretch receptors, we found they powerfully control ending sensitivity; from turning the ending off completely to doubling normal output! We now want to understand why this powerful regulation is needed. Then, we can understand if there are diseases associated with the system that were previously unsuspected, or might benefit from targeting with new drugs. For example, we are now testing if they can help us understand loss of co-ordination in diabetes, or mitochondrial diseases. We are even looking at this same system in stretch-sensitive endings around blood vessels. Might they be a new target for treating the most prevalent cardiovascular disease risk - high blood pressure, also known as hypertension.
How do motor nerve endings work and adapt?
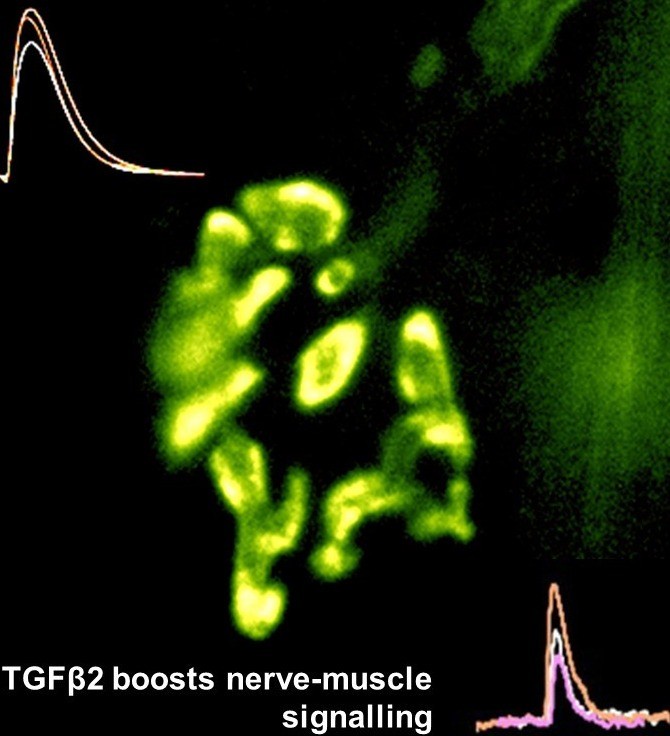
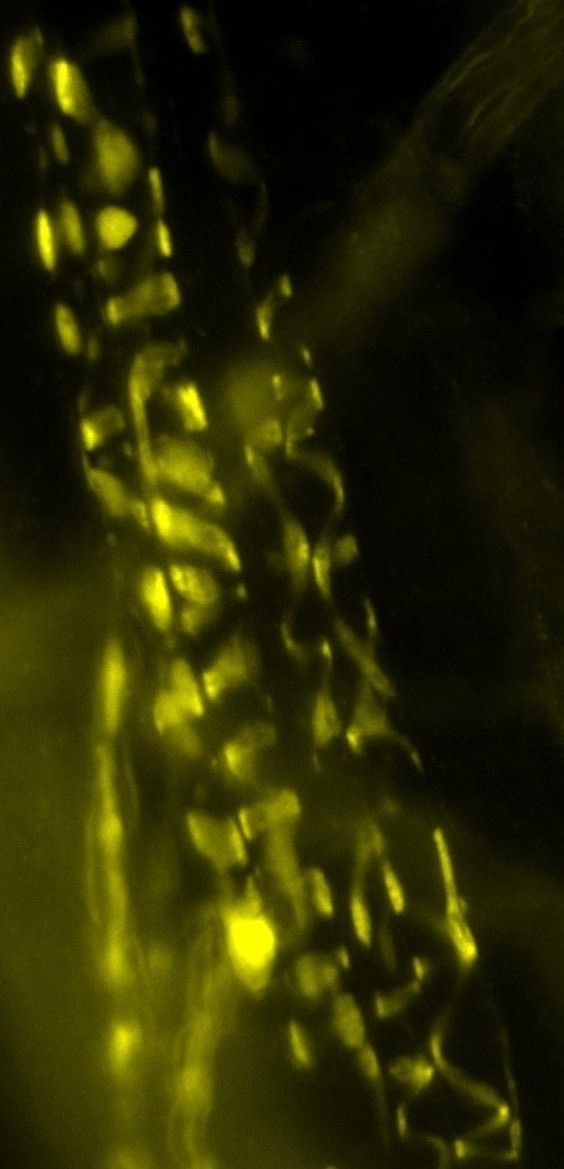
Motor nerve terminal (yellow/green)
and recordings showing TGFbeta2
boosts transmitter release
nerve-muscle signalling.We are studying how motor nerve terminals know what to do to activate their target. When we know that, these mechanisms may be targeted to treat diseases such as motor neurone disease (MND/ALS), or congenital muscle weakness. We have shown that motor nerve terminals on muscle cells 'learn' (i.e. become trained) to change neurotransmitter output, to suit their patterns of use. We want to know how this is done. How does a nerve terminal know how much transmitter to release to get the muscle to respond, even if the muscle cell changes size and how often it is used? How does it know how much neurotransmitter to store, depending on whether it is used often (e.g. marathon runners) or rarely (powerlifters). By understanding these mechanisms, they can become targets to treat motor nerve diseases, such as myasthenia (congenital myasthenia, myasthenia gravis) or sick motor neurones in motor neurone disease (MND/ALS). We found that a protein (TGF-beta2) is secreted by the muscles during activity, causing the nerve terminal to release more transmitter, making it more effective. This is probably one signal between the two cell types to keep signalling 'just right' (not too much, not too little) in healthy muscles as they adapt to changing demands, such as growth, training and ageing. Now we are testing if this system can be targeted by drugs to increase signalling in conditions where nerve-muscle signalling is weakened by disease, such as early stage motor neurone disease, and myasthenias. An important new development is designing special cell culture dishes allowing human cells to be kept in just the right conditions, and to mimic human diseases in culture.
Past Research
1979-1986 (with Dr David Tonge, King's College London, UK).
Reinnervation of mammalian muscle after nerve damage nerves is effective (does occur, and does restore muscle contraction) but is not selective (will innervate the wrong muscle equally well). Conversely, in tailed amphibians, reinnervation by damaged motor nerves is both effective (produces muscle contraction) and specific (innervates only the correct muscle). This likely reflects the axolotl growing continuously, producing new nerves and muscles, that need to recognise each other throughout life.
1988-90 (with Prof Bill Betz, Univ of Colorado, USA).
We developed the technique for using FM1-43 and other styryl pyridinium dyes for visualising how living nerve terminals work during activity. This technique can be combined with a range of other recording techniques, too. It has >1000 citations, and has been adopted by laboratories all over the world. In addition to neuroscience, it has also been adapted for studies of surface vesicle trafficking in yeast, bacteria, plants, endocrine cells, and epithelial cells. We also determined the synaptic vesicles recycle time under continuous activity to be ~100 seconds.
1990-1994 (with Prof Clarke Slater, Univ of Newcastle upon Tyne, UK).
We showed the distribution of muscle proteins correlated with specific parts of the neuromuscular junction. Utrophin is associated with the crests of the postsynaptic folds, along with the acetylcholine receptors, while spectrin and dystrophin are in the depths of the postsynaptic folds, along with the voltage-gated Na channels.
Since arriving in Aberdeen
Motor nerve function:

We found that release from motor nerve terminals is adapted to their patterns use by adjusting the ratio of the number of transmitter quanta (i.e. vesicles = quantal content) released per stimulus, and the size of the vesicle pool (number of vesicles in the terminal). The recycle time was fixed under continuous activity at ~100 seconds. Thus, to maintain release, a terminal needs enough vesicles to last 100 seconds. With Dr Brian Reid (Aberdeen, UK, now UC Davis, USA), Profs Arild Nja, Terje Lomo (Oslo University, Norway) and Clarke Slater (Newcastle upon Tyne, UK).We then found that in the depletion of a neurotrophic factor NT-3, or blockade of the enzyme myosin light chain kinase (MLCK) vesicle trafficking is severely reduced, but transmitter release is much less affected. With Prof Phil Sheard & Marilyn Duxson, Univ of Otago, NZ).
Most recently, we showed that a peptide factor is secreted by the muscle cell, directly under the active motor nerve terminal, called transforming growth factor beta-2 (TGFb2), increases the efficiency of nerve-muscle communication. With Dr Sitt Wai Fong (Aberdeen, UK now Taipei University, Taiwan) and Prof Ian McLennan (Univ of Otago, NZ).
Lab-on-a-Chip (LOC) system to study ALS

Muscle cells awaiting contacts from
stem-cell derived human motor nerve
cells in LOC model of ALS.
(Claire Hetherington, PhD student).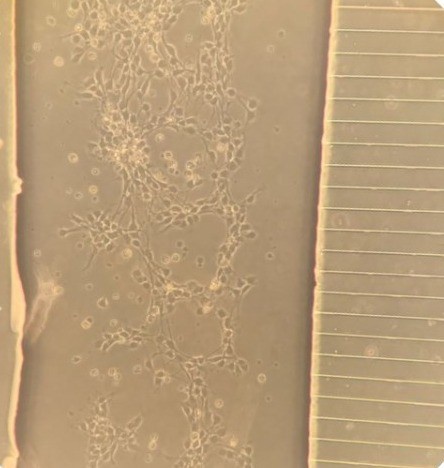
Stem cell-derived motor nerve cells
from ALS patient growing through
cross channels (right) to contact
muscle cells in adjacent chamber.
(Abigail Dos Santos, PhD student)With Dr Claudiu Giuraniuc, we are now developing a Lab-on-a-Chip (LOC) system to study human motor neurone disease in a dish. If successful, we aim to develop a system for rapid assay of drugs to promote survival of motor nerve cells and their muscle contacts in this devastating disease. As each set of stem cells comes from an individual with ALS, this system could also be the basis for personalised treatments for difference types of ALS, and even individual patients. At least, we hope so....
Stretch-sensitive nerve terminal model
Our latest model of how these nerve endings work
Animation produced by Media Services and Medical Illustration, University of Aberdeen.© (2022) Guy Bewick, University of Aberdeen.
Supervision
My current supervision areas are: Biomedical Sciences.
We have one exciting PhD opportunity currently available (self-funding applicants only):
Mitochondria & motor control : nerves detecting muscle length are full of mitochondria. Is this why loss of co-ordination is so common in mitochondrial disease? This project explores mitochondria in terminal function and as potential therapeutic targets.
- Publications
-
Page 3 of 7 Results 21 to 30 of 63
Piezo is essential for amiloride-sensitive stretch-activated mechanotransduction in larval Drosophila dorsal bipolar dendritic sensory neurons
PloS ONE, vol. 10, no. 7, 0130969Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1371/journal.pone.0130969
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/4692/1/Piezo.pdf
The PDZ-Domain Protein Whirlin Facilitates Mechanosensory Signaling in Mammalian Proprioceptors
Journal of Neuroscience, vol. 35, no. 7, pp. 3073-3084Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1523/JNEUROSCI.3699-14.2015
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/4292/1/3073.full.pdf
Mechanotransduction in the muscle spindle
Pflugers Archiv : European Journal of Physiology, vol. 467, no. 1, pp. 175-190Contributions to Journals: ArticlesSynthesis and biological evaluation of (-)-kainic acid analogues as phospholipase D-coupled metabotropic glutamate receptor ligands
Organic & Biomolecular Chemistry, vol. 12, no. 47, pp. 9638-9643Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1039/c4ob02002b
A study of the expression of small conductance calcium-activated potassium channels (SK1-3) in sensory endings of muscle spindles and lanceolate endings of hair follicles in the rat
PloS ONE, vol. 9, no. 9, e107073Contributions to Journals: ArticlesAnalyses of muscle spindles in the soleus of six inbred mouse strains: Spind.les in soleus muscle
Journal of Anatomy, vol. 223, no. 3, pp. 289-296Contributions to Journals: Articles- [ONLINE] http://onlinelibrary.wiley.com/doi/10.1111/joa.12076/full
- [ONLINE] DOI: https://doi.org/10.1111/joa.12076
Glutamatergic modulation of synaptic-like vesicle recycling in mechanosensory lanceolate nerve terminals of mammalian hair follicles
The Journal of Physiology, vol. 591, no. 10, pp. 2523-2540Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1113/jphysiol.2012.243659
- [ONLINE] View publication in Scopus
TGF-β2 alters the characteristics of the neuromuscular junction by regulating presynaptic quantal size
PNAS, vol. 107, no. 30, pp. 13515-13519Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1073/pnas.1001695107
Investigation of neuromuscular abnormalities in neurotrophin-3-deficient mice
European Journal of Neuroscience, vol. 31, no. 1, pp. 29-41Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1111/j.1460-9568.2009.07032.x
A role for glutamate in regulating mechanosensory sensitivity: Further evidence from rat muscle spindle primary afferent terminals
Proceedings of the Physiological Society, vol. 19, C120Contributions to Journals: Abstracts
