What is Bioavailability? - Definition
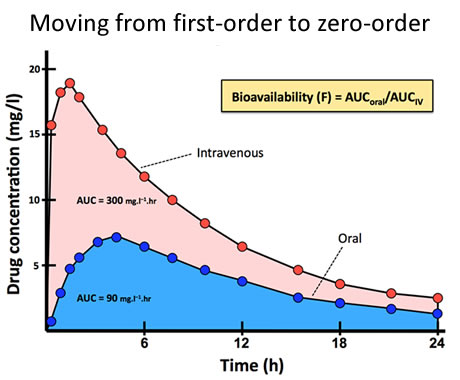
After administration of an intravenous dose, the entirety of that dose enters the systemic circulation. This may not be true after administration by other routes. After oral drug doses only a proportion of the dose reaches the systemic circulation because of incomplete absorption or first-pass metabolism occuring in the gut wall or liver. This proportion is known as the bioavailability, which is assessed by comparing the area under the concentration-time curve (AUC) after an oral dose with that after an intravenous dose (Fig 16). Bioavailability (F), usually expressed as a percentage, is derived from:
Bioavailability is an important concept in drug development because it determines whether a drug is suitable for oral administration and what the range of optimal doses will be. Furthermore, once a drug's bioavailability is known, it can be used to estimate clearance without the need to give an intravenous dose, from the equation:
Some common drugs have a very low oral bioavailability and have to be given by other routes e.g. insulin (broken down by gastric acid), glyceryl trinitrate (first-pass metabolism). Although bioavailibility is often used to denote oral bioavailability the term can also be used to describe the availability of unchanged pharmacologically active drug after administration by other routes.
