What are the Main Concentration-time Relationships? - First-order Kinetics

For drugs eliminated by first-order processes, the decline in plasma concentration is fairly predictable from the equation:
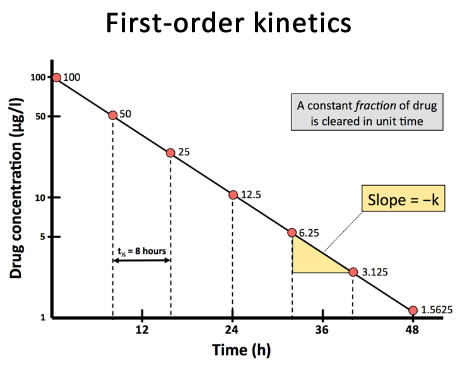
However, the elimination rate constant is not a practical index for prescribers and the rate of first-order processes is usually expressed in terms of the half-life - the time taken for the process to be half completed.
The half-life (t½) is simply related to the elimination rate constant (k) in the following way. After one half-life, the plasma concentration (C0) will have fallen by half, therefore:
... e�kt� = 0.5 (dividing by C0)
... kt� = ln2 (natural logarithmic transformation)
... t� = 0.693/k (natural logarithm of 2 = 0.693)
The half-life remains constant throughout the period of drug elimination, so the time taken for any given plasma concentration to fall by half, at any time after administration, will remain the same (Fig 9). Plotting plasma concentration against time on a logarithmic scale (Fig 10) enables this to be seen more easily.