
Personal Chair
- About
-
- Telephone Number
- +44 (0)1224 438159
- Office Address
Health Services Research Unit
University of Aberdeen
3rd Floor, Health Sciences Building
Foresterhill
Aberdeen
AB25 2ZD- School/Department
- School of Medicine, Medical Sciences and Nutrition
Biography
I am an MRC Senior Non-Clinical Fellow and Professor working in Methodological Research related to participant centred trials.
My research interests focus on the design and delivery of participant centred trials: from point of initial contact through to feeding back results to individuals. I lead research in the application of behavioural science to trials methodology. For example, framing problems of recruitment and retention as behaviours and exploring the opportunities to improve these aspects through the design of participant centred, theoretically informed, interventions. This work is multi-disciplinary and multi-stakeholder and involves using mixed methods approaches to develop, evaluate and implement interventions to support decision making in this context. To find out more about specific project please click on the 'Research' tab at the top of the page. My research has been supported by various funders.

I also lead the mixed-methods process evaluations in many of our trials to improve both the experience for potential participants and the overall efficiency of the trial. Work in this area is ongoing across the portfolio of CHaRT trials.
Visual abstract of our STEER project which used behavioural approaches to develop interventions to address trial retention. Full paper here.

Qualifications
- MSc Public Health and Health Services Research2009 - University of Aberdeen
- PhD Biochemistry2007 - University of Dundee
- BSc (Hons) Biomedical Science2001 - Glasgow Caledonian University
Latest Publications
How should trial teams make decisions about the proportions and diversity of the ethnic groups in their trial?
Trials, vol. 25, 768 (2024)Contributions to Journals: ArticlesLaparoscopic cholecystectomy versus conservative management for uncomplicated symptomatic gallstones: economic evaluation based on the C-GALL trial
British Journal of SurgeryContributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1093/bjs/znae293
What is the carbon footprint of academic clinical trials? A study of hotspots in 10 trials
BMJ Open, vol. 14, no. 10, e088600Contributions to Journals: Articles‘It’s a bit kind of nebulous’:: Unanticipated impacts of patient referral pathways on clinical trial recruitment
Contributions to Conferences: Oral Presentations- [ONLINE] Conference programme
The UK resuscitative endovascular balloon occlusion of the aorta in trauma patients with life-threatening torso haemorrhage: The (UK-REBOA) multicentre RCT
Health Technology Assessment, vol. 28, no. 54Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.3310/LTYV4082
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/24578/1/Jansen_etal_HTA_The_UK_Resuscitative_VoR.pdf
- [ONLINE] View publication in Scopus
Prizes and Awards
MRC Senior Non-Clinical Fellowship 2023-2028. Medical Research Council.
MRC Strategic Skills Methodology Research Fellowship 2014-2019. Medical Research Council.
CSO Postdoctoral Research Training Fellowship 2009-2012. Chief Scientist Office of the Scottish Government.
- Research
-
Research Overview
Trials methodology; behavioural science; behaviour change; participant experience; process evaluations; mixed-methods.
Research Areas
Research Specialisms
- Healthcare Science
- Applied Science
Our research specialisms are based on the Higher Education Classification of Subjects (HECoS) which is HESA open data, published under the Creative Commons Attribution 4.0 International licence.
Current Research
Methodological research related to RCTs
Behavioural approaches to trials methods
I lead several projects (or work packages within projects) that have applied behavioural science to understand key trial problems such as recruitment and retention. These include:
- What are the key challenges and opportunities for mounting a trial of prehospital REBOA? A behavioural diagnosis to inform a definitive evaluation (PPRO_Behave)
- Systematic Techniques to Enhance Retention in RCTs: The STEER Project
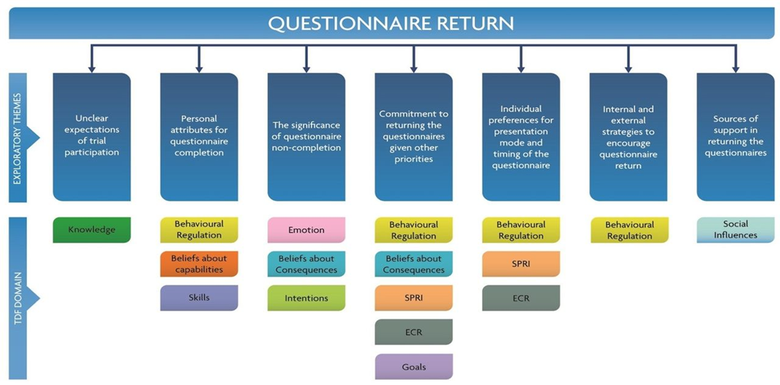
- Behavioural approaches to explore factors that affect questionnaire return in the CGALL trial. The figure below illustrates the overarching themes related to questionnaire return with the relevant TDF domains mapped against each theme.
I also supervise PhD candidates in this area as lead academic supervisor or lead methods expert:
- Behavioural interventions to improve clinical trial recruitment and retention
- Recruiter experience of recruiting pregnant women to clinical trials (The ENCOUNTER Study)
Other recently completed or ongoing trials methods projects that I lead
- Development of a core outcome set for the EvaLuation of Interventions for informed Consent for randomIsed controlled Trials: The ELICIT Study. ELICIT, the first methodological core outcome set, was developed to identify what outcomes should be considered in evaluations of interventions that aim to improve decisions about participation in a clinical trial. The photo above was taken at the final consensus meeting which was held in Aberdeen in February 2020 and brought together a range of stakeholders to help build agreement on the most important outcomes. Full paper here

- PRioRITY II: Prioritising Retention in Randomised Trials – I led a multi-stakeholder James Lind Alliance Priority Setting Partnership to identify the Top 10 unanswered questions for methods research on trial retention. Full paper here.
- Strategies to improve retention in randomised trials. I led the recent collaborative update of this Cochrane review.
- RECAP: Feedback matters: How should trial results be reported back to participants? The RECAP project has generated participant-centred, evidence-based recommendations for trialists to implement the dissemination of results to trial participants. The findings from RECAP are informing the Health Research Authority’s update to dissemination of trial results guidance.

Process evaluations within ongoing RCTs
- REGAL: Recurrence of Endometriosis: A randomised controlled trial of clinical and cost-effectiveness of Gonadotrophin Releasing Hormone Analogues with add-back hormone replacement therapy. Started recruitment in 2021.
- REINFORCE: A Real-World, In-Situ, Evaluation Of The Introduction And Scale-Up Of Robot-Assisted Surgical Services In The NHS: Evaluating Its Impact On Clinical And Service Delivery, Effectiveness And Cost. Started recruitment in 2023.
- PARTIAL: A randomised trial of the clinical and cost effectiveness of PARTIAL vs radical nephrectomy for clinically localised renal cell carcinoma. Started recruitment in 2023.
Recently completed
- C-GALL - A randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of laparoscopic cholecystectomy compared with observation/conservative management for preventing recurrent symptoms and complications in adults with uncomplicated symptomatic gallstones. Methodological research projects that I lead linked to this trial include:
- UK REBOA: A randomised controlled trial of the effectiveness, and cost-effectiveness, of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for trauma. Process evaluation applying a behavioural approach to improve recruitment to the trial through identification of barriers and implementation of behavioural solutions.
Other applied Health Services Research Projects
I-TRAC - In-home Tracking of glaucoma: Reliability, Acceptability, and Cost: the I-TRAC Study (Chief Investigator)
REINFORCE - Real-World Evaluation of Robot-Assisted Surgical Services (REINFORCE): Work Package 1 - Optimisation of RAS implementation and scale up (Methodological lead)
RoboCOS: What outcomes are important for evaluating robotic assisted surgery as a service-level change? An outcome mapping exercise to inform core outcome set development (Methodological lead)
TestES Consortium: Testosterone Effects and Safety in Men with Low Testosterone levels (Methodological lead)
Collaborations
I actively collaborate with other methodologists and trial teams both nationally and internationally on a range of trials methodology projects, highlights include:
- ORINOCO: Optimising Resource-use IN Outcome Collection
- PoINT: Public involvement in Numerical aspects of Trials
- PACT: Patient-centred trials (PACT): developing measures to improve the experience of people taking part in clinical trials
- Developing and Testing Participant Information Leaflets (PILs) that Inform and do not Cause Harm (PrinciPILs)
- BadRaP: Using behavioural science to understand and improve participation in clinical trials
Supervision
My current supervision areas are: Applied Health Sciences.
I supervise PhD candidates at the University of Aberdeen and other Institutions, recent examples include:
- Design and delivery of greener trials - Frank You (MRC DTP Studentship)
-
Can audit and feedback be applied to target healthcare professionals recruitment and retention behaviour in RCTs? A mixed methods exploration - Mojca Cerar (SGSSS DTP studentship)
-
Behavioural Optimisation and Operational Strategies for Trials - Naomi Young
- How do trial teams plan for trial retention? - Ellen Murphy (University College Cork)
- Ryan McChyrstal (University of Glasgow)
- Alice Marie Toader (University of Liverpool)
- Ella Howes (University of Leeds)
Recently completed
- Behavioural interventions to improve clinical trial recruitment and retention - Taylor Coffey
- Recruiting women during pregnancy and childbirth to clinical trials - Vivienne Hanrahan (National University of Ireland, Galway)
Funding and Grants
I have been involved as a co-applicant and work package lead on a portfolio of national and international grants generating in excess of £19 million, with almost £3 million as Chief Investigator, predominantly over the past 5 years. These awards include a range of funders, methodological and applied research, but all are relevant for national or international healthcare. Select awards over past 5 years include:
- Gillies K (CI). Behavioural Optimisation And Operational Strategies For Trials: The BOOST Approach. MRC Senior Non-Clinical Fellowship. 04/23 - 03/28 £1,649,861
- Goulao B, Gillies K, Campbell M, Ramsay C. Patient and public INvolvement In target differeNces in Trials (The PINpoINT Study). MRC New Investigator Research Grant (BG). 10/23-04/26. £534,210
- Gillies K (CI). Implementation of trial methods research: a study of challenges and opportunities -TRiP study. MRC Trials Methodology Research Partnership. 07/22 – 01/23 - £12,855
- Soomro N, Gillies K, Breckons M, Challacombe B, MacLennan G, Vale L, Narahari K, Sheerin N, Stewart G, Nicol D, di Mambro D, Heer R. The PARTIAL study – a randomised trial of the clinical and cost effectiveness of PARTIAL vs radical nephrectomy for clinically localised renal cell carcinoma. NIHR HTA. 02/22-01/27 - £1,876,106
- Gillies K (CI), Duncan E, MacLennan G, LeBrec V, Lendrum R. What are the key challenges and opportunities for mounting a trial of prehospital REBOA? A behavioural diagnosis to inform a definitive evaluation. 10/21-03/22 - £12,000
- Treweek S, Gardner H, Gillies K, Witham M, Devane D, Khunti, Bower P, Parker A, Oshisanya A, Soulsby I. . Improving ethnic diversity in trials: helping trial teams recruit and retain the ethnic groups essential for results with community-wide relevance and applicability. Chief Scientist Office of the Scottish Government’s Health & Social Care Directorate. 11/21-03/23 - £188,146.
- Williamson P, Yap C, Eldridge S, Gillies K, Hughes D, Gates S, Jaki T, Taylor R, Walwyn R, Tudur-Smith C, Vale C, Wason J, Hosking J. Doctoral Training Programme 2021 – Trials Methods Research . Medical Research Council. 09/22-09/26 - £2,518,806
- Beard D, Matthew C, Harji D, McGaughey F, Torkington J, McGrath J, Gillies K, Davies L, Vale L, MacLennan G, Soomro N, Bhattarai N, Bach S, Shaikh S, Campbell MK. A Real-World, In-Situ, Evaluation Of The Introduction And Scale-Up Of Robot-Assisted Surgical Services In The NHS: Evaluating Its Impact On Clinical And Service Delivery, Effectiveness And Cost. NIHR HSDR 01/22-03/25 - £1,359,238
- Howick J, Gillies K, Treweek S, Bower S, Edwards A, Bostock J, Hood K. PrinciPIL: Developing and Testing PILs that do not Cause Harm. MRC Better methods, better research Programme. MRC Better Research, Better Methods Programme. 01/21 - 08/22 - £312,832
- Brehaut J, Presseau J, Gillies K, Grimshaw J, Ramsay C, Duncan E, Fergusson D, Marlin S, Graham I, Spencer H, Weijer C, Taljard M, Gordon J, Richards D, Rodger M. Using behavioural science to understand and improve participation in clinical trials. CIHR. 02/21-02/26 - £551,440
- Gillies K (CI), Azuara-Blanco A, Hernandex R, Forrest M, Maclennan G. Digital technologies for home monitoring glaucoma: a feasibility study. NIHR HTA Programme. 08/20 - 12/22 - £289,313
- Williamson PR, Emsley R, Sydes M, Land T, Brown J, Lalloo D, Mabey D, Hood K, Devane D, Farrell B, Allen E, Kirkham J, Gillies K, Yap C, Blazeby J, Avery K, Weir C, Jaki T, Wason J, Hughes D, Farron A, Morris T, Landray M, Wordsworth S, Villar Moreschi S. Trials Methodology Research Partnership, Medical Research Council. 06/19 - 01/23 - £458,665
- Teaching
-
Teaching Responsibilities
I teach on the Qualitative Methods module for the Masters in Public Health. I supervise Masters students across the MPH and other in-person and online Masters courses. I contribute to the Real World RCTs and PPI in trials modules that HSRU has developed.
- Publications
-
Page 12 of 15 Results 111 to 120 of 144
Exploring non-retention in clinical trials: A meta-ethnographic synthesis of studies reporting participant reasons for drop out
BMJ Open, vol. 9, no. 6, e021959Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1136/bmjopen-2018-021959
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/12378/1/e021959.full.pdf
- [ONLINE] View publication in Mendeley
Prioritizing research areas for antibiotic stewardship programmes in hospitals: a behavioural perspective consensus paper
Clinical Microbiology and Infection, vol. 25, no. 2, pp. 163-168Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/j.cmi.2018.08.020
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/11868/1/Prioritizing_research_areas_for_antibiotic_stewardship_programmes_in_hospitals.pdf
- [ONLINE] View publication in Scopus
- [ONLINE] View publication in Mendeley
- [ONLINE] https://abdn.pure.elsevier.com/en/en/researchoutput/prioritizing-research-areas-for-antibiotic-stewardship-programmes-in-hospitals(92caf58a-15e8-4a8a-bf58-7db15ecc8ac5).html
Surgeons' and methodologists' perceptions of utilising an expertise-based randomised controlled trial design: A qualitative study
Trials, vol. 19, 478Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1186/s13063-018-2832-z
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/11079/1/s13063_018_2832_z.pdf
Relative importance of informational items in Participant Information Leaflets for trials: a Q-Methodology approach
BMJ Open, vol. 8, no. 9, e023303Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1136/bmjopen-2018-023303
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/11064/1/e023303.full.pdf
Patient reported measures of informed consent for clinical trials: A systematic review
PloS ONE, vol. 13, no. 6, e0199775Contributions to Journals: ArticlesSystematic Techniques to Enhance rEtention in Randomised controlled trials: the STEER study protocol
Trials, vol. 19, 197Contributions to Journals: ArticlesTrial Forge Guidance 1: What is a Study Within A Trial (SWAT)
Trials, vol. 19, 139Contributions to Journals: Letters- [ONLINE] DOI: https://doi.org/10.1186/s13063-018-2535-5
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/10095/1/s13063_018_2535_5.pdf
A protocol for a systematic review of non-randomised evaluations of strategies to increase participant retention to randomised controlled trials
Systematic reviews , vol. 7, 30Contributions to Journals: Articles“It’s trying to manage the work”: A qualitative evaluation of recruitment processes within a UK multi-centre trial
BMJ Open, vol. 7, no. 8, pp. 1-8Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1136/bmjopen-2017-016475
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/9151/1/e016475.full.pdf
‘Is that it?’ Using ‘explorachoc’ to engage the public with clinical trials and health services research
Trials, vol. 18, no. Suppl 1, pp. P158Contributions to Journals: Abstracts
