Ongoing
- PINpoINT: Patient and public INvolvement In target differeNces in Trials (The PINpoINT Study)
- CreatStat: Developing and evaluating creative workshops to enhance communication of statistics in patient and public involvement in clinical trials
- Eliciting and incorporating patient’s opinions about missing data in randomised controlled trials. PhD student: Sophie Greenwood
- EPPIC: Experiences of Patient and Public Involvement in target differences in Clinical trials. PhD student: Vitri Darlene
Completed
- How do we know if a treatment is good enough? A survey of non-inferiority and equivalence trials
- INITIAL: Involving patients and the public In sTatistIcal Analysis pLans
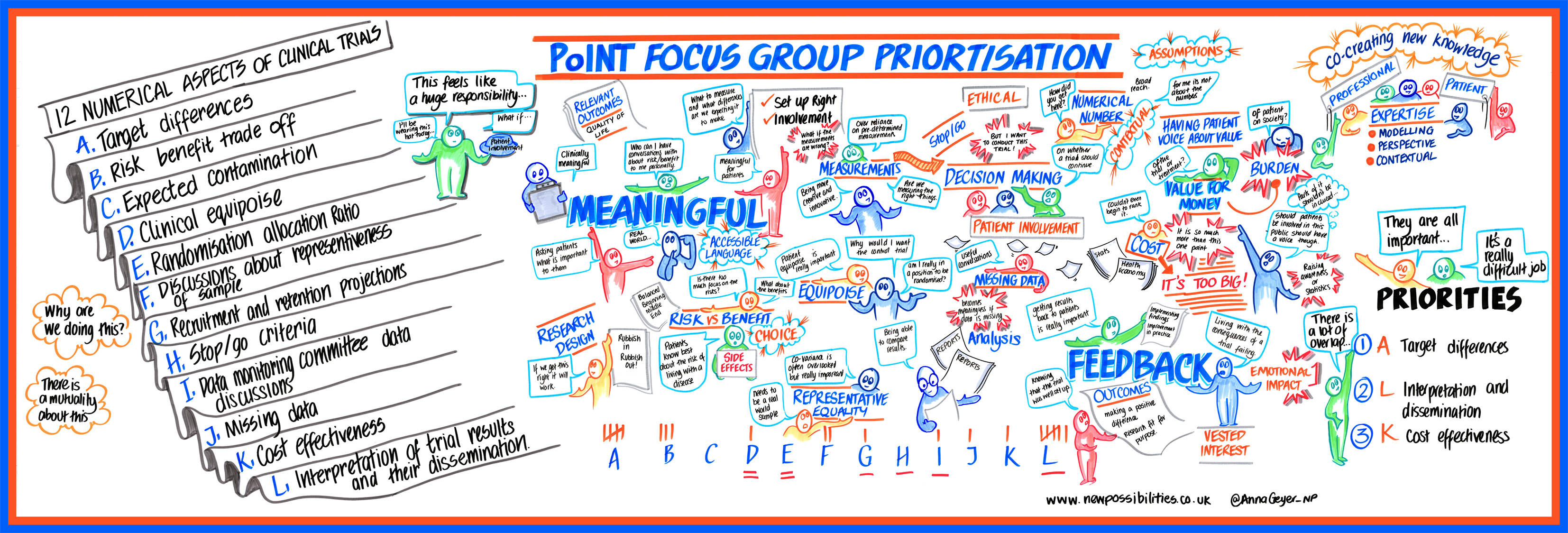
- The original PoINT project: Public Involvement in Numerical aspects of Trials
- Patient and public involvement in numerical aspects of trials: a mixed methods theory-informed survey of trialists’ current practices, barriers and facilitators https://bmjopen.bmj.com/content/11/3/e046977.info
Contacts
- Beatriz Goulao; beatriz.goulao@abdn.ac.uk
Status
OngoingPublications
Goulao B, Bruhn, H, Campbell, M, Ramsay, C, Gillies, K. Patient and public involvement in numerical aspects of trials (PoINT): exploring patient and public partners experiences and identifying stakeholder priorities. Trials 2021.
Goulao B, Poisson, C., Gillies, K. Patient and public involvement in numerical aspects of trials: a mixed methods theory-informed survey of trialists’ current practices, barriers and facilitators. BMJ Open, 2021;11:e046977.
Goulao B, Campbell MK, Gillies K, Ramsay C. What methods are used to involve patients and the public in numerical aspects of research? A scoping review. Trials 20(Suppl 1):579
Attard N, Totton N, Gillies K & Goulao B. How do we know a treatment is good enough? A survey of non-inferiority trials. Trials 23, 1021 (2022). https://doi.org/10.1186/s13063-022-06911-8