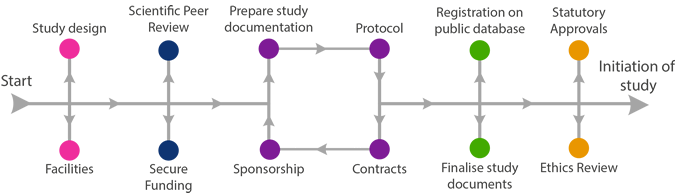
Finalise Study Documents

The following checklist can be used as a guide to which documents may need to be in place before the study can start. If in doubt, check with the Sponsor.
- Confirmation of sponsorship letter
- Final approved study protocol signed by all parties according to local requirements
- Final approved participant information sheet(s) and consent form(s) and GP letter
- Final approved other written participant information e.g. diary card(s)
- Final approved participant recruitment advertisement (if relevant)
- Research ethics committee (REC) approval
- NHS R&D permission
- Clinical Trial Authorisation (CTA)* with any stated conditions addressed (if appropriate)
- Sign off from a statistician (if required)
- Signed off/finalised case report forms (CRFs)
- Signed off/finalised results database (if required)
- Systems for managing safety data (e.g. Serious Adverse Event Reporting)
- Details of any data monitoring committee or trial steering or management group (if not in protocol)
- Standard Operating Procedures all in place
- Investigator's Brochure or Summary of Product Characteristics
- Signed agreements including operational and financial arrangements
- Statement of insurance
- CVs and other evidence of relevant training (e.g. GCP/Regulation/protocol) and qualifications for the investigator(s) and local study team members
- Normal values/ranges for laboratory/medical/technical tests/procedures
- Laboratory accreditation(s)
- Pharmacy documentation/file
- Decoding procedures for blinded trials
- Delegation logs, screening/enrolment logs, participant identification log, randomisation logs (where applicable)
- Trial start-up/initiation report (or confirmation that site initiation activities have been completed.
Version Control
For multi-centre sites it is necessary to synchronise documents across all investigational sites, the Chief Investigator must make sure that each Principal Investigator at each site is provided with all the relevant, version controlled documents before starting recruitment.
A copy of all documents should be taken and filed in the Trial Master File