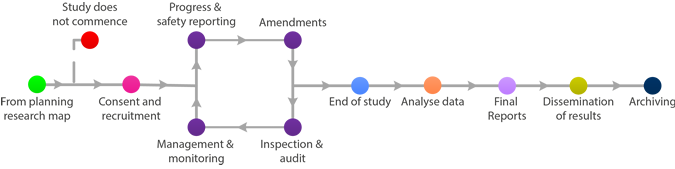
Final Reports
A Clinical Trial Summary Report is generally required to be submitted within one year of the end of study declaration. This should be sent to the Sponsor, MHRA (for CTIMPS) and relevant Ethics Committee and any other stakeholders as required by the protocol.
The summary of the final report may be enclosed with the end of study declaration if complete at that time.
There is no standard format for final reports. As a minimum, information should be provided on whether the project achieved its objectives, the main findings and arrangements for publication or dissemination of the research, including any feedback to participants.