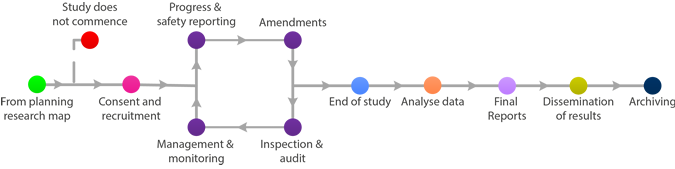
The most common means for the dissemination for results are:
- Journal publications
- Conference presentations
- Copy of report to participants
CONSORT guidelines can be followed when preparing a manuscript for a clinical trial and the SPIRIT statement for study protocols. These ensure that all relevant information is reported in the publication.
The results from all studies should be published regardless of their findings. Research results which are “negative”, i.e. the expected and/or wanted effect was not observed should still be made available to the research community and a wider audience. Newer journals, such as Trials and Journal of Negative Results counter-balance selective reporting by publishing articles regardless of outcome or significance of findings.
Clinical Trials of Investigational Medicinal Products (CTIMPs)
The summary results of all CTIMPs must also be published in the European clinical trials database, EudraCT.
Visit the SOPs and Templates page for further guidance.