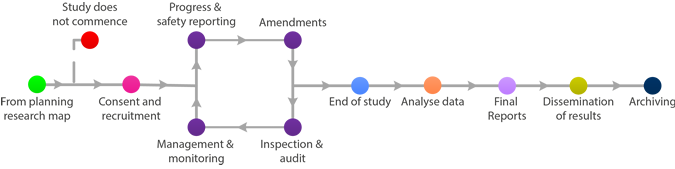
Amendments

Remember that no amendment can be implemented, unless for immediate safety reasons, until all approvals are in place. Until this has happened the study has to continue according to the already approved protocol and related documents.
Instructions on how to process your amendment correctly can be found on our SOP and Templates page.