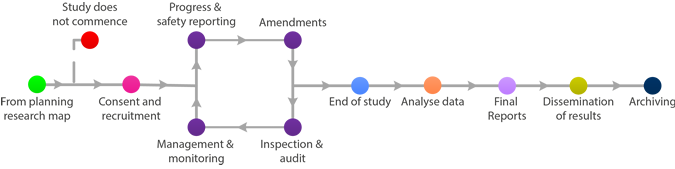
Registration on Public Database

- Clinical trial of an investigational medicinal product (CTIMP)
- Clinical investigation or other study of a medical device
- Combined trial of an investigational medicinal product and an investigational medical device
- Other clinical trial to study a novel intervention or randomised clinical trial to compare interventions in clinical practice.
If your research falls into any of the above categories, you must make sure you register your study BEFORE enrolment of the first patient; otherwise you may not be able to publish.
The only registries that currently meet UoA/NHSG criteria are www.clinicaltrials.gov and the ISRCTN Register (http://isrctn.com ).
Detailed guidance about registration is given in IRAS.