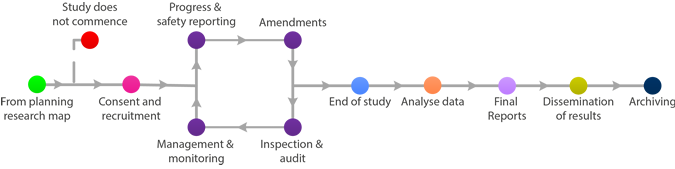
Managing your Research

- Run safely
- Fully recruited
- Completed on time and within budget
- Analysed correctly
- Closed out
- Published
Using the tabs provided, you will be able to identify key points in the active phase of research.
Remember: Any changes to your approved study MUST be approved by your Sponsor first.