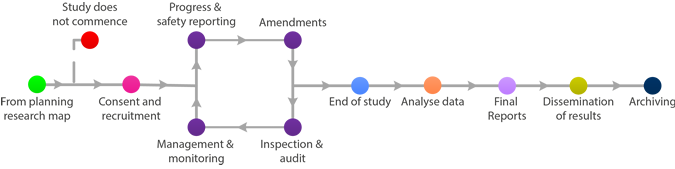
End of Study
The following list can be used to help cover things that may need to be considered, dealt with and closed out before end of study but check with the Sponsor for the trial to ensure that you have covered everything:
- Declaration of End of Trial
- Analysis of samples
- Analysis of data
- Destruction of unused and/or returned IMP
- Transfer of custody of any remaining tissue samples to appropriate persons /biobank (if consent in place) or disposal
- Confirm archiving procedures for electronic data
- Check that patient confidentiality remains in place and will be retained
- Collection of all documents retained elsewhere whilst study was actively recruiting (i.e. Sample Logs)
- Check of all essential documents in the Trial Master File
- Fulfil all obligations to participants i.e. information re trial results, notification of results to GP
- Custody transfer or return of any trial specific equipment
- Publication and dissemination of results
- End of trial report (within 1 year of End of Trial Declaration)
- Close out visit by monitor
For CTIMPS the end of the study will be pre-determined in the research protocol, normally considered to be the last visit of the last participant, and the End of Trial Declaration will need to be completed, but this is not the end of a researcher's obligations and in many cases the EoT is completed prior to analysis of data.
Visit HRA website for further advice and guidance
Visit the SOP page for further details