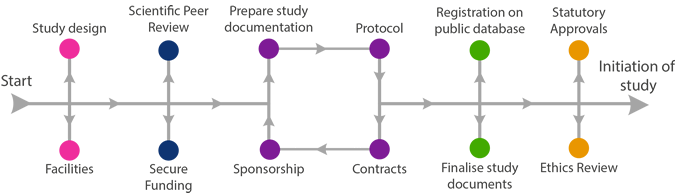
Statutory Requirements

Clear evidence of the documents submitted and the documents that were approved need to be retained in the Trial Master File.
Once proof of funding is in place, application can be made for sponsorship approval.
For Clinical Trials of Investigational Medicinal Products (CTIMPS) and High Risk Studies:
- Research Governance carries out an initial risk assessment, agrees it with the Investigator, and study is sent to the Clinical Studies Oversight Group for full risk assessment and confirmation of sponsorship arrangements
- Research Governance notifies the Investigator when sponsorship approved (including insurance cover)
- Investigator sends IRAS forms to MHRA, REC and R & D and any other required regulatory body
- Investigator send copies of ALL responses to Research Governance
- Sponsor (green light) approval is given when ALL approvals are in place