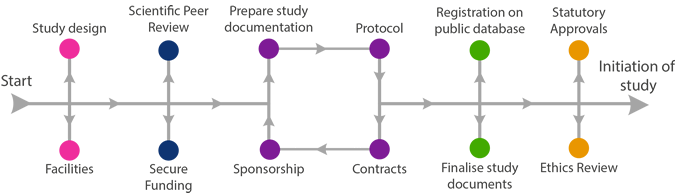
NoSRES RECs give their opinion about the proposed participant involvement and whether the research is ethical. The majority of research conducted within the NHS requires ethical review. Contact Research Governance at your earliest opportunity for guidance. If a full NoSRES REC opinion is not required, your research may be reviewed instead by an internal ethics committee such as SERB (SMMSN Ethics Review Board) or by the Rowett Institute of Nutrition & Health or School of Psychology ethics committees.
The NoSRES REC will review:
- Relevance of trial
- Trial design
- Risks and benefits
- Protocol
- Suitability of the investigator and supporting staff
- Investigator brochure if required
- Quality of the facilities
- Subject information
- Consent procedure
- Justification for including minors or adults unable to give informed consent
- Insurance/ indemnity
- Rewards or compensation for investigators and subjects
- Subject recruitment
- Registration on Public Database
Provisional sponsorship approval MUST be in place before application is made to the NoSRES REC.
Applications to a NoSRES REC can only be made through the Integrated Research Application System (IRAS).
Research projects which raise no material ethical issues may apply for NoSRES REC approval using the Proportionate Review Service (PRS). These applications are reviewed by a sub-committee rather than the full NoSRES REC committee with decisions usually being made within 14 days of receipt of the application.
Ethics Committees will consider researcher's plans for Patient and Public Involvement (PPI) as part of the ethical review process. N.B. Specific ethical approval does not need to be sought when involving the public in trial design and management activities.