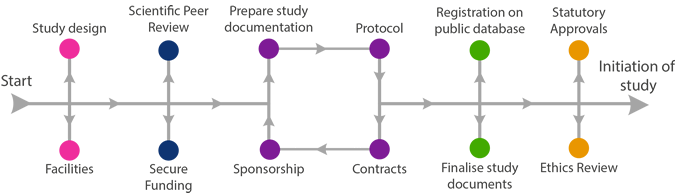
Acceptance of funding award
Please notify Research & Innovation (R&I) when your study has been funded. R&I will arrange acceptance of the award, as well as any necessary research agreements.
What agreements will be required for my study?

- Funding Agreement;
- Collaboration Agreement;
- Third Party Supply Agreement;
- Sub-contracts e.g. laboratory services;
- Data Processing Agreement or Data Sharing Agreement.
In addition, for medicinal product/device trials and other interventional studies:
- Co-sponsorship Agreement;
- Drug Supply Agreement;
- Technical Agreement;
- Site Agreements.
For CTIMPs and Medical Device Clinical Investigations please refer to the current Generation of Contracts SOP
Involvement of third party service providers & suppliers in clinical studies
Statutory procurement processes must be followed; requirements will vary based on the value of goods or services and R&I can help ensure required procurement rules are followed.
The University - NHSG Grampian Quality Assurance Manager (QAM) may undertake a third assessment ahead of signing an agreement with a supplier. Please refer to the current SOP for the Selection and Management of Third Parties and contact the UoA-NHSG QAM as soon as a third party suppliers have been identified.