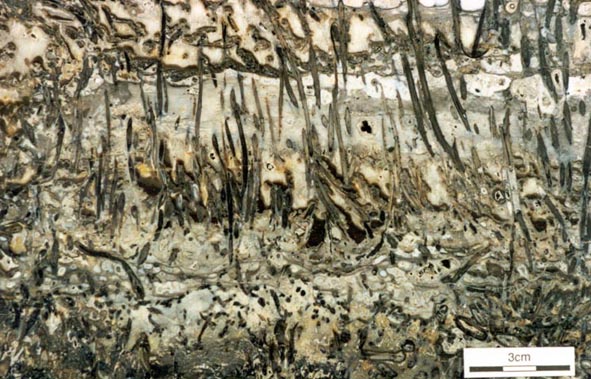
The early land plants found as fossils in the Rhynie chert are locally preserved in such exquisite detail that cellular details can be examined. This has allowed detailed anatomical studies to be performed on the Rhynie plants. The plants are relatively simple in their level of organisation and include seven identified 'higher land plants', two enigmatic nematophytes and a number of other plants including various types of fungi, algae and the earliest fossil lichen.
We can demonstrate that at least seven of the plants are true subaerial plants by most or all of the following features being preserved:
- Cuticle with cutan that helps preserve moisture.
- Stomata for gaseous exchange with the atmosphere.
- Intercellular air space network for gas diffusion.
- A vascular strand with lignin for water conduction and support.
- Sporangia with a well developed dehiscence ('splitting') system to release spores.
- Spores
The taxonomy of the Rhynie plants poses difficulties for subdivision into currently accepted taxonomic groups. For the purposes of this resource, we have made a simple subdivision into the 'higher land plants' - those with the features listed above, and 'other non-vascular plants' from the chert.
Higher Land Plants
The seven higher land plants of the Rhynie chert 'macroflora' that have been described to date are detailed below. Various life stages have also been described for a number of the plants with both the sporophyte and gametophyte stages having been identified (e.g.: Remy & Hass 1986, 1991a,b,c,d; Remy & Remy 1980a,b; Remy et al. 1993 and Kerp et al. in press). A number of these plants exhibit other delicate features such as mycorrhizae, bacterial infections and various forms of pathological damage. Five of the plants are true vascular plants or tracheophytes, showing tracheids in the water-conducting cells. Two plants, Aglaophyton and Nothia do not show tracheids and can therefore not be considered as tracheophytes.
- Basic information on the higher plants in the Rhynie chert
-
There are seven genera of terrestrial macroplants described from the Rhynie chert. Of these, five are considered to be true vascular plants, displaying tracheids in the water-conducting tissue, but the status of Aglaophyton and Nothia, which appear to lack tracheids, remains in doubt. Rhynia and Aglaophyton are the most abundant, Asteroxylon, Nothia and Horneophyton fairly common, and Trichopherophyton and Ventarura scarce. These plants seldom exceed 20cm in height.
Aglaophyton major

The water-conducting cells in this plant did not have thickenings like true vascular plants, being more similar to those seen in some modern bryophytes (a group including mosses and liverworts). The simply branched naked stems are more comparable with the extinct rhyniophytes and as such its systematic position remains unknown.
Aglaophyton grew mainly on dry, litter covered, organic-rich substrates, on its own as monotypic stands or with other Rhynie plants, though it seems to have required wet conditions for germination.
Asteroxylon mackiei

In cross-section the water-conducting strand of this plant forms a characteristic star-shape pattern from which smaller strands radiate to meet the base of the 'leaves'.
Asteroxylon is a member of a group of plants called the lycophytes which includes modern club mosses.
Asteroxylon mainly grew in organic-rich soils as part of a diverse community together with other Rhynie plants and could probably tolerate quite dry habitats.
Horneophyton lignieri

The water-conducting cells in this plant, like true vascular plants, possessed thickenings. However, the presence of a collumella in the sporangia shows similarities with bryophytes (a group including mosses and liverworts). As such the systematic position of this plant remains uncertain.
Horneophyton preferred to grow on sandy and organic-rich substrates, often on its own as monotypic stands and probably flourished in damp to wet conditions.
Nothia aphylla

The water-conducting cells in this plant did not have thickenings like true vascular plants, being similar to those seen in some modern bryophytes (a group including mosses and liverworts). The stalked, lateral kidney-shaped sporangia are comparable with zosterophylls and the simply branched naked axes with rhyniophytes and as such its systematic position remains unknown.
Nothia preferred to grow in sandy soils and plant litter, on its own or with other plants.
Rhynia gwynne-vaughanii

Rhynia is a member of an extinct group of primitive plants called the rhyniophytes, characterised by their simple branching and naked stems.
Rhynia commonly grew in thickets, typically on its own as monotypic stands and was often an early coloniser of well-drained sinter and sandy substrates. It also grew with other plants and was tolerant of a wide range of habitats.
Trichopherophyton teuchansii

The water-conducting cells in this plant, like true vascular plants, possessed thickenings. This together with the shape and position of the sporangia suggest Trichopherophyton belongs to a group of plants called the zosterophylls.
Trichopherophyton was a late coloniser of organic-rich substrates, always growing with other Rhynie plants as part of a diverse flora.
Ventarura lyonii

The water-conducting cells in this plant, like true vascular plants, possessed thickenings. This together with the shape and position of the sporangia suggest Ventarura belongs to a group of plants called the zosterophylls.
The palaeoecology of Ventarura is not fully known, but it probably grew in localised patches, at least in the vicinity of freshwater ponds and probably in sandy and organic-rich substrates.
- Detailed descriptions of the individual genera and their palaeoecology
-
Each of the panels below has more detailed descriptions of the individual genera and their palaeoecology.
Aglaophyton
Introduction
Aglaophyton was originally described as Rhynia gwynne-vaughanii by Kidston and Lang in 1917. In 1920, however, they split the genus into two species and assigned this plant to the species Rhynia major. D. S. Edwards (1986) re-interpreted the plant, based primarily on the lack of thickenings on the xylem cells (a feature originally attributed to poor preservation by Kidston and Lang (1920a) ) and renamed the plant Aglaophyton major. Both the male and female gametophytes of Aglaophyton have been identified though, to date, only the male gametophyte, Lyonophyton rhyniensis, has been formally described and named (Remy & Remy 1980b). The overall morphology and palaeoecology of Aglaophyton is outlined below.
Morphology
'Aerial' Axes

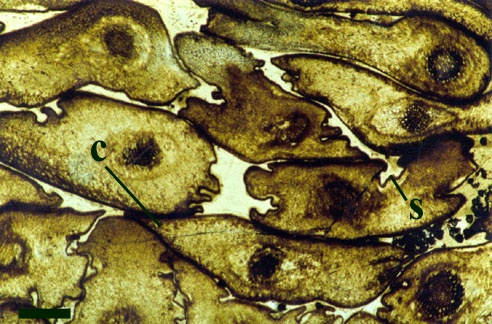
The epidermis of Aglaophyton is smooth and the cuticle appears deeply flanged. The stomata are flanked by two reniform or kidney-shaped guard cells. Most of the axis comprises the cortex (see inset right), this is divided into two, the division occasionally marked by a distinct brown layer.


Rhizomal Axes

Sporangium

The disposition of the sporangia is terminal and they usually occur in pairs above the last point of dichotomy (see reconstruction below).
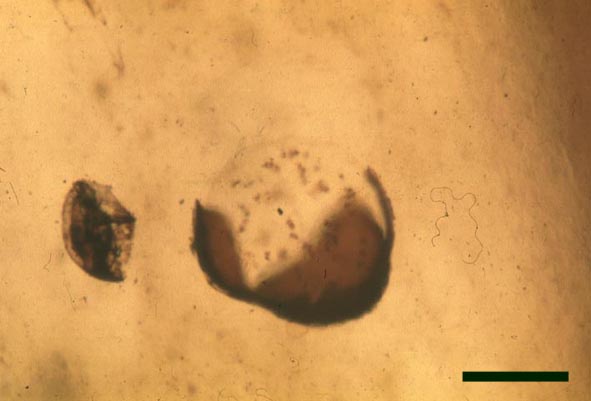
The spores of Aglaophyton are retusoid and have smooth walls with a trilete mark located in a thinning of the exine. They are relative large, ranging in diameter from 64μm to 85μm. These spores are comparable to species of the spore genus Retusotriletes. The spores of Aglaophyton have often been found preserved at various stages of germination (Lyon 1957) (see the section on spores for images of some of these).
Gametophytes


Reconstruction
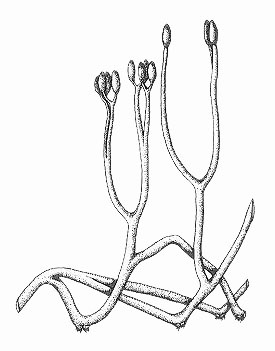

Relationships
The systematic position of Aglaophyton, like a number of other Rhynie plants, remains unresolved because it shows a mixture of anatomical and morphological features that are not typical of any one group of plants. It shows many features characteristic of the rhyniophytes, a group of primitive plants, known only from fossils, showing simply branched naked stems of which Rhynia is an example. The water-conducting cells of the xylem strand do not show thickenings and as such are more reminiscent of the hydroids of some bryophytes (a group of plants including mosses and liverworts). Since the xylem of Aglaophyton does not possess true tracheids, this suggests it is not a true vascular plant.
Palaeoecology
Aglaophyton was a common plant and significant component of the Rhynie ecosystem during the Early Devonian. It appears to have preferred growing on litter-covered, organic-rich surfaces and was never a primary coloniser of sinter substrates, though the creeping rhizomal axes of Aglaophyton probably allowed it to occupy large surfaces of substrate. It occasionally grew as monotypic stands but is often seen associated with other plants, particularly Nothia, Asteroxylon, Horneophyton and occasionally Rhynia.
Since Aglaophyton exhibits stomata on the rhizomal axes as well as the upright aerial axes, it seems likely that the plant colonised mainly dry substrates. The fact that the cuticle and stomata of Aglaophyton display adaptations to prevent water loss suggests it could also tolerate periods of drought (Powell 2000b).
However, the matrix of chert beds containing Aglaophyton in growth position occasionally contain the charophyte Palaeonitella, in association with the freshwater crustacean Lepidocaris and the 'aerial' axes of the plant may display infestation by chytrids (chytridomycetes) which are a type of tiny, simple fungi that thrive in damp and especially aquatic conditions. Another feature noted by Remy and Hass (1996) is the association of germinating Aglaophyton spores with these aquatic elements. Although the plant could probably also tolerate humid or damp conditions, this does not necessarily mean that Aglaophyton lived in standing water, perhaps these particular beds represent instances where areas colonised by the plant were occasionally flooded. It does seem likely, however, that wet conditions may have been necessary for spore germination.
Asteroxylon

Introduction
Asteroxylon, one of the better known Rhynie plants, was originally described by Kidston and Lang (1920b) and assigned the name Asteroxylon mackiei. They noted fertile elements probably belonging to Asteroxylon within their material; however, the fertile elements of the sporophyte were not finally resolved until Lyon (1964) discovered sporangia in organic connection with the plant and he concluded that the fertile axes observed by Kidston and Lang were in fact of another new plant, Nothia aphylla. To date the gametophytes of Asteroxylon remain unknown. The overall morphology and palaeoecology of Asteroxylon is outlined below.
Morphology
Aerial Axes

One of the characteristic features of Asteroxylon are the scale like enations that emerge from the epidermis in a spiral arrangement around the axes. These are not true leaves since they do not possess a vascular strand (see inset right). The cuticle on both axes and enations bear abundant stomata with distinctive dark-coloured guard cells. The surface of the epidermis of the enations varies from smooth to papillate whereas that of the axes is smooth.
The cortex may be divided into a narrow outer zone of closely packed cells and a broader inner cortex that can be further subdivided into three zones: an outer and inner layer of compact cells with a trabecular middle layer of elongate cells with a well-developed inter-cellular air space network. Occasionally the inner zones of the cortex display fungal infestation (see insert below right).
The vascular strand is quite distinctive. Asteroxylon possess an actinostele, in other words the vascular strand appears star shaped or stellate in transverse cross-section (see insert below left and heading photograph). The xylem is exarch to locally mesarch where the protoxylem occurs at the ends of the 'lobes' of the actinostele, and displays spiral thickenings (see insert below right). Phloem tissue is best developed between the 'lobes' of the xylem strand. 'Leaf traces' are often seen where vascular tissue splits from the central stele. These traces end at the bases of the enations.



Rhizomal Axes

Sporangium

Reconstruction


Relationships
Asteroxylon is rather more anatomically complex than the other known Rhynie chert plants. The plant is considered a true lycophyte (a group of plants which includes the 'club mosses') based on the structure of its apical meristem (Hueber 1992) together with the characteristic exarch actinostele and the lateral disposition of the sporangia. The spirally thickened and reticulate cell walls of the xylem cells are also typical of lycophytes (Kenrick & Crane 1991). Modern lycophytes are also characterised by their enations or microphylls which possess a single vascular strand. In Asteroxylon the vascular trace stops at the base of the enations which may suggest the plant represents an intermediate stage in the evolution of lycopsid leaves.
Palaeoecology
Asteroxylon is quite commonly encountered in a number of chert beds and apparently formed a significant component of the Rhynie flora during the Early Devonian. Its rhizomes are usually found traversing plant litter and the plant is primarily found to occur in situ with two or more other genera, commonly Nothia, Rhynia and Aglaophyton and occasionally Ventarura. It seems therefore that Asteroxylon primarily lived as part of a diverse plant community rather than as monotypic stands (Powell et al. 2000b).
The fact that Asteroxylon possesses an extensive, substrate-penetrating 'root' system suggests it was capable of exploiting larger volumes of water and nutrients than the other Rhynie plants. Also the presence of microphylls increase the surface area of the aerial axes. This would have created a larger photosynthetic surface and with the greater density of stomata the plant possesses would also have meant greater efficiency in gaseous exchange and transpiration. It is likely that Asteroxylon could tolerate quite dry habitats compared with most of the other Rhynie flora.
Horneophyton

Introduction
Horneophyton was originally described by Kidston and Lang (1920a) and assigned the name Hornea lignieri, however, the generic name was already occupied and it was therefore renamed Horneophyton lignieri by Barghoorn and Darrah in 1938. Although one of the better known Rhynie plants, it's systematic position is still very much debated. As well as the sporophyte, the female gametophyte of this plant, Langiophyton mackiei, has also been described (Remy and Hass 1991c). The overall morphology and palaeoecology of Horneophyton is outlined below.
Morphology
Aerial Axes


The aerial axes of Horneophyton display a maximum diameter of 2mm and are cylindrical and naked. The plant probably reached a maximum height of 20cm. The branching of Horneophyton is dichotomous and repeated.
The cuticle of the epidermis displays a regular arrangement of elongate cells. Stomata are relatively rare and are enclosed by distinctly modified cells (Hass 1991) (see inset right). The cortex is generally poorly preserved and where present appears to be undivided.
The vascular tissue comprises an endarch xylem strand with irregular spiral and reticulate thickenings. This is surrounded by a zone of thin-walled cells interpreted as phloem. The vascular strand becomes less distinct towards the base of the aerial axis (see inset below right).
Rhizomal Axes

Occasionally, fungal activity is evident in the cortex of the rhizome (see inset below left).


Sporangium

One of the more curious features of the sporangia of Horneophyton is the presence of a central columella in the sporangial cavity (see inset right).
The in situ spores of Horneophyton are relatively well known (Bhutta 1973a) and may be assigned to the spore species Emphanisporites decoratus. These vary between 42 and 54μm in size, the proximal face bearing a distinct trilete mark. The proximal face of the spore is also ornamented with radial ribs; the distal face characteristically showing an apiculate ornament, typically of closely spaced micron-sized spines.
Gametophytes

Reconstruction


Relationships
The presence of a sterile central columella in the sporangia of Horneophyton is a feature in extant plants seen only in some bryophytes (e.g. mosses) suggesting the plant may have some affinity with the latter, however, the fact that the aerial axes of the plant exhibit a well-developed vascular strand with tracheids would suggest it is not part of the bryophyte lineage. This mixture of features seen in Horneophyton, as with other Rhynie plants, has led to much debate on the systematic position of the plant which still remains in some doubt.
Palaeoecology
Horneophyton appears to have been one of the more common plants in the Early Devonian ecosystem at Rhynie. Where found in situ and in life position, Horneophyton often occurs at the base of composite chert beds, it's subterranean corm-like rhizomes cutting through pre-existing plant litter and it's rhizoids probably helped to anchor the plant. It therefore appears that Horneophyton preferred sandy and organic-rich substrates. The plant is commonly present as monotypic stands, perhaps indicating it was also an early coloniser of sinter surfaces being able to tolerate environmental conditions unfavourable to many other Rhynie plants (Powell, et al. 2000b). For example the plant is seldom associated with Rhynia gwynne-vaughanii in growth position suggesting the two plant taxa required different optimum conditions for growth.
It is also likely that Horneophyton flourished in damp to wet conditions (Powell, et al. 2000b; Remy & Hass 1991c). This seems likely for two main reasons. Firstly, looking at modern sinters, their hygroscopic nature generally means they easily retain water and in some cases the sinter surface where 'early colonising' plants are growing is covered by an intermittent film of water (see also the section on The Ancient Environment & Modern Analogues). Secondly, Remy and Hass (1991c) noted that Horneophyton was often associated with chytrids (chytridiomycetes) which are a type of tiny, simple fungi that thrive in damp and especially aquatic conditions (see also the sections on Fungi under Other Non-Vascular Plants and Evidence for Plant/Animal Interactions).
Nothia

Introduction
Nothia was originally described as the probable fertile region of another Rhynie plant, Asteroxylon mackiei by Kidston and Lang (1920b). This was assumed to be the case until Geoffrey Lyon discovered the unequivocal fertile leafy shoots belonging to Asteroxylon (Lyon, 1964). In the same paper Lyon gave a preliminary description of this enigmatic new vascular plant and assigned it the name Nothia aphylla though no diagnosis was given. El-Saadawy and Lacey (1979b) wrote a more detailed account of Nothia and were the first to provide a diagnosis. The most recent work, concentrating on the rhizomal anatomy, has been written by Kerp et al. (2001).
The systematic position position of Nothia remains unresolved. As well as the sporophyte, the male gametophyte of this plant, Kidstonophyton discoides, has also been described (Remy & Hass 1991b). The overall morphology and palaeoecology of Nothia is outlined below.
Morphology
Aerial Axes

The epidermis of Nothia shows a characteristic pattern of longitudinally orientated files of short cells alternating with 'giant cells' (Kerp et al. 2001) (see inset right). It displays elliptical to lenticular stomata-bearing emergences up to 350μm comprising longitudinal files of short cells with up to four intervening 'giant cells'; the files of short cells bearing the stomata. The pores of the stomata are extremely narrow and their two guard cells are usually wider than long. The cortex is generally poorly preserved (see inset below right) though an inner and outer cortex can occasionally be differentiated based on cell shape (Edwards et al., in press), being short in the outer cortex and elongated in the inner cortex. Below the epidermal emergences the intercellular spaces of the outer cortex are large and within the emergences the outer cortex appears spongy (Kerp et al. 2001).

Rhizomal Axes

The morphology of epidermal and 'vascular' cells of the rhizome are very similar to that of the aerial axes. The epidermis again consists of alternations of files of short cells and 'giant' cells and the xylem strand exhibits small cells surrounded by larger water-conducting cells. Again the xylem cells do not show partial thickenings and thus have a fibrous appearance. The main differences between the rhizomal and aerial axes is the lack of 'emergences' (the axes consequently appearing 'smooth'), lack of stomata and the thickening of walls in the short cells of the epidermis.
Sporangium

Gametophytes

Reconstruction



Relationships
Nothia aphylla is yet another Rhynie chert plant whose taxonomic relationship remains unclear, exhibiting morphological and anatomical features characteristic of a number of plant groups. Firstly it shows features characteristic of the bryophytes, namely the unthickened water-conducting cells (a feature also seen in the 'hydroids' of Aglaophyton major), a feature which suggests it is not a true vascular plant. Secondly, Nothia also shares features with the primitive rhyniophytes since its axes are naked and show similar simple branching. Also the sporangia of this plant show similarities with zosterophylls, being lateral in their disposition on the axes, reniform in shape with a well-developed marginal dehiscence mechanism.
Palaeoecology
Nothia is a relatively common plant in a number of chert beds occurring in allochthonous and autochthonous plant litter, though, with the exception of rhizomal axes, not commonly preserved in growth position. In a few horizons rhizomes and aerial axes of Nothia occur as monotypic assemblages. In most cases the rhizomal axes of Nothia appear to be preserved in cherty sandstone beds along with plant litter. The presence of rhizoids and lack of stomata on the rhizomal axes suggest the rhizomes of Nothia were subterranean, apparently preferring sandy soils.
In some beds Nothia rhizomes appear to have penetrated earlier Nothia rhizomes or the rhizomes of other plants (e.g.: Asteroxylon) which also suggests they grew within plant litter. The rhizomes or primary axes are always far better preserved and less decayed than the aerial axes, even in the few instances where the latter are found in situ in vuggy and massive cherts that usually contain very well preserved axes of other Rhynie plants. These observations tend to suggest a much longer lifespan for the rhizomal (subterranean) axes than the aerial axes the growth of which was perhaps determined by seasonal changes (Kerp et al. 2001).
Rhynia

Introduction
One of the first Rhynie chert plants to be described and perhaps the most abundant is the form Rhynia. The plant was originally described and classified by Kidston and Lang in 1917, 1920a and assigned the species name Rhynia gwynne-vaughanii. Another plant now known as Aglaophyton, possessing a slightly similar anatomy, was originally described by Kidston and Lang in their 1917 paper as also belonging to Rhynia gwynne-vaughanii, but differs significantly in its vascular anatomy and was subsequently reassigned (see also Aglaophyton in the top panel). Unequivocal gametophytes of Rhynia have recently been discovered (Kerp et al. in press) and will be illustrated here once published. The overall morphology and palaeoecology of Rhynia is outlined below.
Morphology
'Aerial' Axes



The stomata typically appear circular on the cuticle surface and are flanked by two guard cells (see inset above right). The cells of the cuticle often exhibit a median ridge giving the cuticle a flanged appearance.

Rhizomal Axes
Sporangium
Reconstruction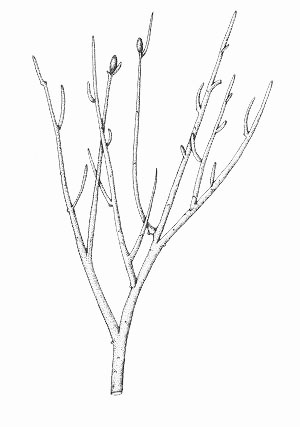
Relationships
Rhynia gwynne-vaughanii being a naked, simply branched sporophyte has been assigned to a primitive group of vascular plants known only from fossils, and called the rhyniophytes.
The presence of hemispherical projections on the axes remains a point of speculation and their like is not seen in any of the other Rhynie plants. Various interpretations have been proposed:
- Damage from arthropods sucking sap (Kevan et al. 1975).
- Wounding by nematodes, mites, parasites or fungi (Edwards and Selden 1993).
- Damage from splashes of hot water from geysers, or from volcanic ash (Kidston and Lang 1921a); an unlikely explanation considering the small size and disposition of the projections on the plant axes, and also since they have not been observed on any of the other Rhynie plants.
- Pant (1962) and Lemoigne (1968a, b) interpreted the hemispherical projections as sites of archegonia , though this interpretation has never been generally accepted.
Palaeoecology
Rhynia was the most common vascular plant in the Early Devonian ecosystem at Rhynie, at least in the areas of sinter deposition, both numerically and in terms of ground cover (Powell et al. 2000b). The plant appears to have been entirely subaerial with its naked, branching 'rhizomes' creeping across the ground surface with the upright portions of the plant growing to give a thicket-like appearance. Although the hemispherical projections on the 'rhizomes' bear the rhizoids for taking up water from the ground surface, the fact that those on the upright stems occasionally exhibit rhizoid tufts suggest Rhynia was also capable of taking up atmospheric water.
Where found in growth position in the chert beds it is typically, though not exclusively the only in situ vascular plant present, and is commonly found above sandy chert layers and allochthonous litter horizons. This suggests Rhynia commonly grew as monotypic stands, an early colonizer of well-drained sinter and sandy substrates.
However, the plant is also found associated with all other Rhynie plants, though only very rarely with Horneophyton. This suggests Rhynia was tolerant of a wide range of habitats and could also withstand interspecies competition within the Early Devonian ecosystem at Rhynie (Powell et al. 2000b).
Trichopherophyton

Introduction
Trichopherophyton is one of the more recent additions to the Rhynie chert list of vascular plants. It was formally described by Lyon & Edwards (1991) and assigned the species name Trichopherophyton teuchansii, and was the first true zosterophyll recorded from the cherts. This is the scarcest of the Rhynie plants having only been found in a few beds of chert.
A reconstruction of the whole plant has not yet been attempted and the gametophyte stage of the plant remains unknown. The overall morphology and palaeoecology of Trichopherophyton is outlined below.
Morphology
Aerial Axes
The aerial axes of the plant exhibit a maximum diameter of 2.5mm. The branching of Trichopherophyton is both dichotomous and pseudomonopodial and it is the only plant known in the Rhynie flora to display circinate vernation.


The vascular tissue comprises a very distinct xylem strand (see inset above right). It is sub-terete, exarch and displays both annular and spiral thickening. A narrow zone, uniform in thickness, of thin-walled cells surrounds the xylem strand and probably represents phloem (see inset above right).
Rhizomal Axes
Observed within many of the chert specimens containing the spinose aerial axes of Trichopherophyton are other generally more common axes of a slightly differing but distinctive morphology that are believed to represent the rhizomes of the plant although there remains no unequivocal evidence for organic continuity between the two.

They exhibit a generally smooth epidermis, lacking the spinose outgrowths though epidermal cells may be papillate and occasionally may bear blunt-tipped emergences that may represent rhizoids. The cortex is quite narrow and less distinctly divided compared with the aerial axes, though again cells of the inner cortex commonly show dark coloured residues (see inset right). A zone of thin-walled cells surrounding the xylem strand may represent phloem. As with the aerial axes the sub-terete xylem strand is very distinctive being exarch and showing narrow annular and spiral thickenings (see inset right).
Sporangium
The sporangia or fertile elements of Trichopherophyton have been observed in a few specimens but have not as yet been fully described. However, sporangia do appear to be reniform (kidney-shaped) with dimensions up to a maximum 3.7mm by 2.5mm. They are attached laterally to the aerial axes with a vascularised sporangial stalk though their spatial distribution is uncertain. The sporangia appear to be bi-valved with a marginal dehiscence mechanism. Characteristically the sporangia display the same unicellular spinose projections emerging from the epidermis as seen in the aerial axes.
The in situ spores (see panel below) of Trichopherophyton appear to vary between 55 and 80μm in size, are retusoid and smooth walled and display a triangular thickening associated with the trilete mark. These spores are comparable to species of the spore genus Retusotriletes.
Relationships
Trichopherophyton teuchansii is undoubtedly a zosterophyll, fitting into the classification of Banks (1975), showing lateral, reniform sporangia with a well-developed marginal dehiscence mechanism as well as an exarch xylem strand with thickenings. However, there remain other features, although characteristic of this plant, that are rarely encountered in true zosterophylls; namely a sub-terete xylem strand and the presence of unicellular spiny epidermal projections on the aerial axes and sporangia.
The circinate vernation seen exclusively in Trichopherophyton is a feature that is also observed in most extant ferns. This suggests that the plant has a more advanced anatomy than that of the other Rhynie plants.
Palaeoecology
Generally Trichopherophyton appears to be relatively rare in comparison with the other vascular plants and has been found to occur in only a few beds of chert. This may partially reflect the scarcity of the plant within the original ecosystem or may simply reflect sampling bias. However, within the few beds within which it occurs, it is preserved both in autochthonous growth position and as litter, but always as part of a diverse flora including Nothia, Horneophyton and locally Rhynia. It seems likely therefore that Trichopherophyton was a late coloniser of humus-rich substrates (Powell et al. 2000b).
Ventarura

Introduction
Ventarura is the most recently identified element of the Rhynie flora. The plant was formally described by Powell et al. (2000a) and assigned the name Ventarura lyonii. The float blocks from which the plant were described all originated from the Windyfield chert of the Rhynie hot spring complex. Many thin sections made of this material have revealed details of the subaerial and probable rhizomal axes, the pattern of branching, and the morphology of the sporangia of the plant.
A preliminary reconstruction of the sporophyte stage of the plant has recently been attempted (see below), though the gametophyte stage of the plant remains unknown. The overall morphology and palaeoecology of Ventarura is outlined below.
Morphology
Aerial Axes

The cortex of the aerial axes of Ventarura is quite distinctive. An inner and outer cortical layer is separated by a dark middle cortex zone of sclerenchymatous cells (see inset below left). The outer cortex comprises closely packed thin-walled cells. The inner cortex again comprises thin-walled cells but with a well-developed inter-cellular air space network.
The vascular strand of Ventarura comprises a generally poorly preserved outer zone of uniform width, tentatively interpreted as phloem tissue. The xylem strand is terete and exarch with annular and spiral thickenings (see inset below right).


Rhizomal Axes

These axes branch repeatedly and tend to exhibit unicellular peg-like outgrowths from the epidermis (interpreted as representing rhizoids) and characteristically lack the sclerenchymatous middle cortex seen in the aerial axes.
Sporangium

Their spatial distribution is not fully known but at least appear to form in a vertical sequence along the fertile axes (see inset top right in section on 'aerial axes').
The sporangia are bi-valved with a well-developed marginal dehiscence mechanism (see inset right).
Preliminary reconstruction

In the sketch the individual sections of the plant that could be traced in the block are darkly shaded, and reveal a variety of branching styles are present.
Numbered features on the reconstruction are:
1. Branched sections of aerial axes traced within a chert block.
2. Sporangia in ?2 regular en echelon rows on axes of 2-3 mm diameter. Sporangia up to 4 mm wide.
3. Branched horizontal rhizomes attached to aerial axis.
4. Rhizomes penetrating bacterial meshworks and decayed aerial axes in water. Growth of the plant probably extended into floating bacterial mats, resulting in vertical penetration of rhizomes into water underneath the mat.
5. Portions of aerial axes were preserved in an inverted position after floating in pools adjacent to growing areas (see heading photograph).
We are still seeking the connections between the fertile axes seen in thin sections and the main aerial axes, also further information on rhizome branching, sporangial arrangement, and the terminations of aerial axes and rhizomes. The search continues prior to formal publication!!
Relationships
Ventarura exhibits a number of features that assist in classification; particularly the morphology of the xylem tissue, and the morphology and disposition of the sporangia. The xylem strand is characteristically exarch with thickenings. The sporangia are reniform to pear-shaped with a well-developed dehiscence mechanism, and have a lateral disposition on the aerial axes to which they are attached by a vascularised sporangial stalk. These features indicate Ventarura is undoubtedly a tracheophyte allied with the zosterophylls and as such is the second zosterophyll known from the Rhynie chert (see also Trichopherophyton in the panel above).
Palaeoecology
To date Ventarura has only been found in a number of chert float blocks from pods of the Windyfield chert. Although locally very well preserved, much of the vegetative and fertile material appears to be at least partially transported, and deposited in freshwater pools evidenced by many of the branched aerial axes being inverted (see heading photograph). The chert texture is typical of silicified subaqueous deposits, containing the crustacean Lepidocaris, microcoprolites and bacterial coatings on plant debris.
As such it remains difficult to sufficiently interpret the palaeoecology of this new plant but with the presence of apparently in situ rhizomatic axes in the chert blocks it seems likely that the plant probably grew at the edges of or at least within the vicinity of these pools and may have preferred sandy and organic-rich substrates.
- Spores
-
Apart from the fossil plants in their own right, fossilised spores are also found, not only in the chert but also in the associated sediments, particularly the shales and mudstones. Many species have been identified and described and have been useful for biostratigraphic purposes in dating the sediments (see section on Age of the Rhynie Chert). There remains, however, a degree of uncertainty as to which of the vascular plants each belongs, and there may well be spores present from other plants that have not yet been found preserved in the cherts.
Rhynie Spores
Fossilised spores from vascular plants are quite common in beds of the Rhynie chert and also within the finer grained interbedded sediments such as shales and mudstones. They are usually extracted from the sedimentary rocks by macerating rock samples in solutions of Hydrofluoric acid which dissolves the silica minerals in the sediment and leaves the resistant spores and other acid-insoluble organic remains for analysis (Wellman & Axe 1999). Interestingly, upon extraction the spores often appear better preserved within the mudrocks than in the cherts, though this may be a reflection of the effects of silicification and replacement of the original organic material.
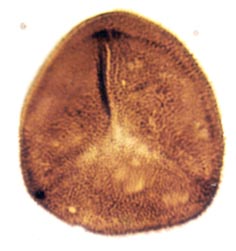

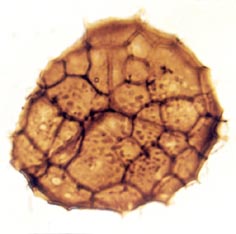


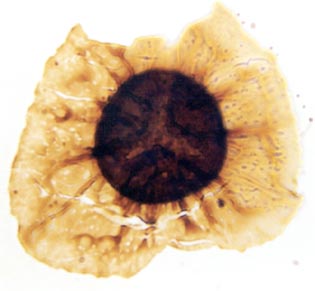
Many genera and species have been recognised and described, though there still remains a degree of uncertainty as to which of the known vascular plants of the Rhynie chert they can be assigned to. In situ spores have been described in the past for Horneophyton and Rhynia by Bhutta (1973a, b). There may well be spores present that are representative of other plants that have not been so far discovered in the cherts. The abundance and variety of spores present in the sequence of Rhynie sediments has been useful in terms of biostratigraphy in determining the age of the rocks (Wellman 2004).
The images above left and right display a few of the spore types or palynomorphs that have been found.
The study of the fossil spores or palynology of the Rhynie chert and sediments is ongoing at the University of Sheffield.
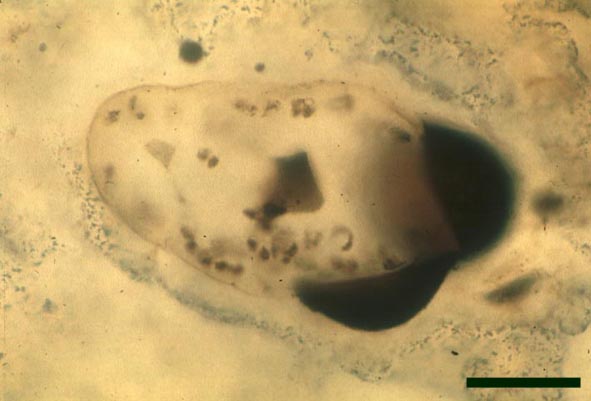
In the Rhynie chert many spores have also been found preserved during various stages of germination. This feature was first described by Lyon (1957). The following four images below are some examples of stages in germination of Retusotriletes (see also insets above right), these are spores of Aglaophyton major.


Other Non-Vascular Plants
The described flora of the Rhynie chert also includes non-vascular plants such as nematophytes, algae, fungi and a lichen.
- Basic information on the remaining plant groups in the Rhynie chert
-
A number of other flora have been described from the Rhynie chert, including the enigmatic nematophytes, cyanobacteria, various types of algae (including simple filamentous and unicellular chlorophytes, and stoneworts or charophytes). Various types of fungi are also present, including terrestrial and aquatic types; the earliest recorded lichen has also been described.
Nematophytes

Two nematophytes are known from the Rhynie chert: Nematophyton taiti (Kidston & Lang 1921b) (see inset right) and Nematoplexus rhyniensis (Lyon 1962). Both are generally fragmentary and poorly preserved.
The gross morphology of nematophytes is not fully known, though some Carboniferous types are believed to have resembled prostrate logs, perhaps with fronds. Their internal structure show similarities with certain algae, and the spirally thickened tubes resemble the tracheids in vascular plants.
The habitat of nematophytes is also not fully known, but they may have been semi-aquatic plants with emergent fronds.
Cyanophytes

A number of probable cyanobacteria are found in the Rhynie chert, some contributing to distinct stromatolitic laminae in the laminated cherts, possibly having originally grown as cyanobacterial mats on sinter surfaces. Other types are found within 'clotted' chert textures, being deposited in more aquatic settings, and still others within decaying plants (see inset right).
Some of the Rhynie cyanobacteria, such as Archaeothrix (inset right), possessed heterocysts and therefore probably played a significant roll in fixing atmospheric nitrogen into the soil.
Chlorophytes

A number of filamentous and unicellular chlorophytes are known from the Rhynie chert, particularly in chert beds deposited in aquatic environments, though very often the poor preservation of cell contents makes their identification very difficult (see inset right).
Charophytes

One probable charophyte has been described from the Rhynie chert, Palaeonitella cranii (Kidston & Lang 1921b). The reproductive structures of Palaeonitella have not been discovered and therefore its status has not been fully resolved.
Palaeonitella was an aquatic plant, being commonly found within 'clotted' chert textures, along with the crustacean Lepidocaris.
Fungi

Numerous fungi are recorded from the Rhynie chert (Taylor et al. in press), including the earliest best preserved examples of endotrophic mycorrhizae in plant tissue (Taylor et al. 1995b) (see inset right). The earliest ascomycetes (true fungi that produce their spores within a sack-like structure called the ascus) are also present (Taylor et al. 1999, in press); together with various tiny aquatic or soil living chytridiomycetes or chytrids. A number of the latter have shown evidence of parasitism on other Rhynie plants and even on other fungi (an interaction called mycoparasitism).
Lichens

The earliest lichen is recorded from the Rhynie chert, Winfrenatia reticulata (Taylor et al. 1995, 1997) (see inset right). Winfrenatia most likely colonised hard substrates. Degrading sinter surfaces could have provided a suitable substrate. It may have been able to weather the rock surfaces it was colonising, thus contributing to soil formation.
- Detailed description of the other forms of plant fossils in the Rhynie chert
-
Each of the panels below has a more detailed description of the other forms of plant fossils in the Rhynie chert.
Nematophytes

Introduction
Nematophytes or the Nematophytales are an extinct group of enigmatic plants known only from Devonian and Carboniferous sedimentary rocks. Their systematic position is unresolved, showing certain affinities with fungi, algae and also, tentatively, with vascular plants. Their gross morphology and habitat (particularly as to whether they were aquatic, semi-aquatic or fully terrestrial plants) are also not fully known, but some appear to have been cylindrical organisms up to 1 metre in length (see Stewart 1999). Nematophytes appear to generally comprise networks of intertwined spirally coiled tubular cells showing both smooth and spirally-thickened walls (the latter similar in appearance to tracheids in vascular plants), with branching localised in distinct 'knots'. The plants appear to have had a variably preserved cuticle-like layer on their outer surface. Certain Carboniferous nematophytes appear to have attained quite a large size, in life probably looking like prostrate logs.
At Rhynie, sandstone casts of probable nematophytes have been found in the past in the Quarry Hill Sandstone. Two incomplete nematophytes have been described from the Early Devonian Rhynie chert: Nematophyton taiti (Kidston & Lang 1921b) and Nematoplexus rhyniensis (Lyon 1962). Fragmentary nematophyte remains also occur in the Windyfield chert (Fayers & Trewin in press). For the purposes of this resource, the morphology of one of the Rhynie nematophytes, Nematoplexus rhyniensis is outlined below:
Morphology
Nematoplexus rhyniensis


The tubes show two distinct morphologies. The most abundant form are smooth-walled, non-septate tubes ranging from 7µm to 10µm in diameter, typically appearing as lax intertwined coils (see inset below left). Branching of the smooth-walled tubes occurs within the smaller branch-knots.
The second type are thin-walled tubes displaying conspicuous spiral thickenings within the wall layer. These range from 2µm up to 28µm in diameter and are again non-septate (see inset below right). The spirally thickened tubes are generally found as short isolated fragments or as irregular clusters within the smooth-walled tube plexus. Branching of these tubes occurs within the larger branch-knots. Occasionally tubes of both types may originate from a single, large branch-knot, though no organic connectivity between the two types have been observed.


Palaeoecology
As stated in the introduction, the habitat of Nematoplexus and nematophytes in general remains unresolved.
A number of authorities believe that nematophytes were aquatic plants. In the Rhynie chert the two nematophytes Nematoplexus and Nematophyton are typically found in association with filamentous green algae (chlorophytes), charophytes, cyanobacteria, occasionally with the crustacean Lepidocaris and with coprolites in a 'clotted' chert matrix; indicative of silicification in a freshwater, aquatic environment. However, in these instances, it may be that the nematophyte remains had been transported, after death, into such aquatic settings or alternatively had been drowned in situ after a flooding event.
They may, however, have been terrestrial or at least semi-aquatic organisms, suggested by the resemblance of the spirally-thickened tubes seen in the Rhynie chert nematophytes to the thickened tracheids in the xylem of vascular plants. It may be that these organisms grew in shallow water with the spirally-thickened tubes differentiated in emergent fronds or 'leaves'. However, to date, no unequivocal evidence of such differentiation has been found in any fossil nematophytes.
Cyanophytes


Introduction
Cyanophytes are an enigmatic group of prokaryotic organisms that comprise what were formerly known as the 'blue-green algae'. Strictly speaking these are only termed 'algae' because they are organisms that can undergo photosynthesis. They are in fact a large and varied group of bacteria, the cyanobacteria, that possess a type of chlorophyll (chlorophyll a) and are able to photosynthesise in the presence of air and sunlight and produce oxygen. Cyanobacteria, unlike chlorophytes, do not possess chloroplasts. In fact the chloroplast in the cells of eukaryotic algae and plants is actually a symbiotic cyanobacterium. In cyanobacteria the chlorophyll is carried on specialised membranes within the cells called thylakoids (see inset below).

Many cyanophytes are important in the fixation of atmospheric nitrogen. This process can only occur in the absence of oxygen, therefore certain cyanobacteria possess specially thickened cells or heterocysts which contain an anaerobic microenvironment in which this can take place (see inset below). Cyanobacteria may be unicellular or filamentous and may or may not be colonial (see inset below).

Modern cyanobacteria live in many different environments ranging from marine to freshwater habitats and also in soil, on rocks and on plants. A few types of cyanobacteria have a symbiotic relationship with fungi forming lichens.
Certain cyanobacteria help to form rigid biogenic structures such as pisoliths and stromatolites. Both are formed by the organisms growing on a particular surface or substrate to which they gradually bind consecutive layers of very fine sediment and precipitated minerals, eventually forming (especially in stromatolites) a stacked sequence of smooth, wavy and crenulated laminae, often forming mounds. Pisoliths usually form by growth around a moveable object such as a sand grain or shell fragment which may be periodically turned over by current action thus creating an almost concentric layer of laminae.
Stromatolites and pisoliths are locally common in the geological record and in certain areas of the world are being formed today such as at Shark Bay in western Australia. Thermophyllic cyanobacteria also produce stromatolitic textures in hot spring areas where they bind detrital grains and precipitated opaline silica to the substrate (see inset below, and section on The Ancient Environment and Modern Analogues).

Fossil Record
Cyanobacteria comprise the earliest forms of life, their fossils having been found in Archaean Precambrian rocks in western Australia dated at almost 3500 million years old. Some of the best preserved fossil cyanobacteria have been found in cherts (including the Early Devonian Rhynie chert) such as in the Late Proterozoic Bitter Springs Chert in Australia. In many cases it is the structures formed by these organisms, primarily pisoliths and stromatolites, that are often preserved in the fossil record, particularly in limestones and carbonate rocks. Both are common and locally well-developed, for example, in the Carboniferous limestones of Ireland and Derbyshire in England and also in the Upper Jurassic Purbeck Limestones of southern England.
The Early Devonian Rhynie chert yields a number of fossil micro-organisms and sedimentary textures that may be attributable to cyanobacteria. Most of the latter occur as well-developed stromatolitic structures that are highly comparable with those formed by the growth of cyanobacterial mats on modern sinter terraces and in hot springs (see heading photograph and sections on Chert Textures and The Ancient Environment and Modern Analogues). However, because of the paucity of well-preserved diagnostic features, only a few probable cyanobacteria have been formally described and named by Kidston and Lang (1921b), Croft and George (1959) and D.S. Edwards and Lyon (1983); Archaeothrix contexta, Archaeothrix oscillatoriformis, Kidstoniella fritschii, Langiella scourfeldii, *Rhyniella vermiformis and Rhyniococcus uniformis. Many other unicellular and multicellular forms of probable cyanobacterial origin are also present but for the lack of sufficient diagnostic features have not been formally described and named.
For the purposes of this resource we will concentrate on the genus described by Kidston and Lang (1921b), Archaeothrix, the morphology of which is outlined below.
*Note: For the species of probable cyanobacteria described by Croft and George (1959), Rhyniella vermiformis, the generic name was already occupied by the collembolan Rhyniella praecursor.
Morphology
Archaeothrix

Neither of these cyanobacteria are especially common; A. oscillatoriformi has been found within partially decayed stems of Rhynia (see inset right) and A. contexta has been occasionally found as large masses lying loose within the chert matrix.
Chlorophytes

Introduction
Chlorophytes are a particular division of eukaryotic algae comprising the green algae. They are characterised by containing two types of chlorophyll, a and b and starch is formed in chloroplasts (see inset below). Green algae may be unicellular but can also form complex multicellular structures such as that seen in the stoneworts or charophytes (see section on charophytes below). These non-vascular plants are typically found in freshwater environments.

Fossil Record
Chlorophytic algae are among the oldest known fossils, being recorded from Precambrian rocks primarily from the Ediacara fauna. These Ediacaran fossils are acritarchs and were probably formed by unicellular algae. In fact the discovery of undoubted chlorophytes in the 850 million year old Late Proterozoic Bitter Springs Chert in Australia was the first ever evidence of early eukaryotes. Multicellular chlorophytes are first seen in Cambrian strata where they helped to build algal reefs. Very well preserved chlorophytes are also known from the famous Middle Cambrian Burgess Shale in British Columbia, Canada.
Apart from 'charophytes', a number of other chlorophytes have been found in the Rhynie chert including unicellular and filamentous types. Of these, to date, two filamentous types have been formally described by D.S. Edwards and Lyon (1983); Mackiella rotunda and Rhynchertia punctata. The morphology of these simple green algae is outlined below.
Note: It must be remembered that the affinities of many fossil algae, including those found in the Rhynie chert remain uncertain. This is primarily because modern classifications of algae are based on biochemical and ultrastructural features which are rarely or indeed never preserved in the fossil record (D.S. Edwards & Lyon 1983). Also, positive identification may be compounded by the variable preservation of cell contents.
MorphologyMackiella rotunda
This alga comprises unbranched filaments up to 850µm in length forming an unattached thallus with no rhizoids. The filaments consist of up to 25 cylindrical cells, each cell typically being more-or-less equal in length and in width (23µm to 41µm and 28µm to 40µm respectively). The terminal cells of the thallus are rounded and slightly longer than the other cells (29µm to 49µm in length). The cell walls are thin and do not show a mucilaginous sheath. The cell contents comprise fine granular material and a dark spherical body, 3.5µm in diameter, interpreted by D.S. Edwards and Lyon (1983) as a pyrenoid or the chloroplast.
Mackiella was a eukaryotic alga. Because the alga shows unattached filaments that may fragment into short filaments and the cells possess a single pyrenoid, D.S. Edwards and Lyon (1983) tentatively assigned Mackiella to the extant order Ulotrichales.
Rhynchertia punctata
This alga consists of an unbranched, unattached thallus comprising multicellular filaments of cells 8µm to 17µm in width and commonly twice that in length. The terminal cells of the thallus are rounded and slightly shorter than the other cells. The cells are thin walled and do not show a mucilaginous sheath. The cell contents may be uniform, or with a single dark body (possibly a chloroplast) or may contain many small ovoid bodies. The latter have been interpreted to be reproductive elements, possible zoospores or gametes (D.S. Edwards & Lyon 1983).
Rhynchertia filaments have been found in association with the nematophyte Nematoplexus, primarily within 'gelatinous' areas where the latter appears to have degraded (Lyon 1962). Based on gross morphology, this alga has also been tentatively assigned to the Ulotrichales by D.S. Edwards and Lyon (1983).
Charophytes

Introduction
Charophytes or stoneworts are one of the largest and most structurally complex of the green algae, and are thought to be part of the evolutionary lineage that lead to vascular plants (Kenrick 1994). As green algae, charophytes are sometimes classed within the Chlorophyta but are often classed as a distinct group, the Charophyta. These plants are aquatic, and modern forms are found in many freshwater to brackish habitats such as ponds, lakes, lagoons and streams. The main axes of mature plants comprise a series of multicellular nodes interspersed by relatively long single cells or internodes. Branches and branchlets grow from individual nodal cells in a whorled arrangement (see inset below). The gametangia, or fertile elements of the plant are quite characteristic in charophytes. The female gametangium or oogonium is formed from a number of divided cells that grow out from one of the nodes; the outer most cells are elongate and spiral around the oogonium forming a protective sheath (see inset below). The male gametangium or antheridium also grows from a nodal cell and is a complex spherical structure usually comprising eight shield-cells, each one enclosing a stalked cell bearing tiny rounded head-cells which in turn bear filaments comprising the cells which produce the spermatozoids. The position of the gametangia on the plants is variable with different charophyte species and can be diagnostic.

As fossils, charophytes can be quite in sediments deposited in suitable environments. It should be noted that the oogonia may be allochthonous or easily transported, but in instances where the axes of the plant are also preserved it is likely these are more-or-less in situ or autochthonous.
Fossil Record
Charophytes are quite well represented in the fossil record from the Tertiary, Cretaceous and Jurassic where they are locally abundant, particularly in limestones and marls deposited in brackish or freshwater settings. In these rocks it is usually the fossilised oogonia that are found (commonly called gyrogonites). This is because the oogonia are often calcified and are easily preserved as a mineral of calcium carbonate, typically calcite. In some instances, for example in the Upper Jurassic/Lower Cretaceous Purbeck Limestones of southern England, fossil charophyte oogonia are so common, diverse and widespread they can be used as zone fossils for biostratigraphy (e.g. Feist et al. 1995). The preservation of whole plants is not common, but they can be quite exquisite in their detail (e.g. Martín-Closas, C. & Diéguez 1998).
The earliest fossil charophytes have been recorded from the Upper Silurian, though whether or not these are true charophytes is still debated. Early Devonian charophytes have been found exquisitely preserved in the Rhynie chert and were first recorded and described by Kidston & Lang (1921b). The Rhynie charophytes show many similarities with the extant charophyte group, the Nitelleae, and have been assigned to the species Palaeonitella cranii. The morphology of Palaeonitella is outlined below.
Morphology
The morphology of Palaeonitella is relatively simple, its structure being closely comparable to that of modern Nitelleae (see inset above). An entire reconstruction of the alga has not been published, though much of the plant is known, primarily the branch whorls, rhizoid nodes and possible bulbils. The status of Palaeonitella has been questioned by Tappan (1980), but recent discoveries of fertile elements in the chert indicate unequivocally that the plant is a charophyte alga (Kelman et al. in press). The fertile elements will be figured here once published.
Axes
The overall size of the plant is not known, but it comprises upright primary axes that bear regularly spaced clusters of nodal cells with whorls of lateral branches. The spacing of nodal cells or rather the length of the internodes does, however, tend to decrease towards the distal end of the axes (see inset below left). These branches in turn may bear secondary branches. The number of branches or rays in a whorl can be variable. Whorls commonly bearing up to ten secondary rays have been observed on the lateral branches. The primary axes are slender generally being up to 200µm in diameter, lateral branches are conspicuously narrower being up to 100µm in diameter with very fine secondary branches being up to 50µm in diameter (see inset below right). Occasionally the nodal and internodal cells appear hypertrophic; mutated and swollen where they have been infested by aquatic fungi (Taylor et al. 1992).


Rhizoids and Bulbils

Palaeoecology
Palaeonitella was undoubtedly an aquatic plant. It is typically found in chert associated with other aquatic biota, particularly crustaceans, chlorophytic algae and chytrids (tiny aquatic fungi). The enclosing matrix commonly contains coprolites and exhibits a clotted or 'mulm-like' texture. It is likely that the alga was an early coloniser, primarily living in still, relatively shallow freshwater temporary ponds, apparently with rather soft silty and organic-rich substrates. If Palaeonitella had a similar ecology to that of most modern Nitelleae it is likely the waters it inhabited were generally quite alkaline. Most extant charophytes tolerate waters with a range of between pH 6 and pH 9. In a few charophyte-bearing chert horizons, many of the specimens preserved in situ exhibit an orientation with axes appearing to be swept or bent over in a particular direction. This probably represents current alignment (Fayers and Trewin in press); maybe Palaeonitella could also tolerate areas of slow moving water, perhaps colonising rather sheltered areas in the cool distal reaches of run-off channels from hot springs.
Fungi

Introduction
Fungi are an important group of multicellular eukaryotic organisms comprising a meshwork of thread-like filamentous cells joined end-to-end. The whole body of a fungus is called the mycellium. Fungi are usually classified into four main divisions; the chytridiomycetes or chytrids (tiny, predominantly aquatic fungi), the zygomycetes (bread molds and mycorrhizae), the ascomycetes (terrestrial, mostly saprophytic fungi, including cup fungi and yeasts), and the basidiomycetes (club fungi, including toadstools and mushrooms; entirely terrestrial); the differences made primarily on the process of reproduction and the morphology of the sporangia since the reproductive structures are generally more diverse than the mycellium.
- Chytrids have motile sexual and asexual spores with posterior flagella.
- Zygomycetes have thick-walled resting sexual spores called zygospores, their asexual spores are produced in a sporangium and called sporangiospores.
- Ascomycetes produce sexual spores (ascospores) in a sack-like body called an ascus, their asexual spores are produced externally, borne on a conidiophore.
- Basidiomycetes produce their spores externally from a club-like structure called a basidium, these generally have no asexual spores.
Fungi may reproduce sexually or asexually and like plants show alternations in their life cycle.
Fungi are unable to build structural materials by photosynthesis (they are heterotrophic organisms). They live on organic material from other living things, alive or dead and are therefore generally parasitic or saprophytic. Some types of fungi, however, form symbioses with plants. Certain fungi are mutualists forming symbioses with a green alga (chlorophyte) or a cyanobacterium to create lichens. Some types of symbiotic zygomycetes live within plants and are called endotrophic mycorrhizae (literally "fungus-root"). The latter are fungal hyphae that grow into the cells of plants, branching within them to form arbuscles where the exchange of nutrients takes place. The hyphae also occasionally grow to form thick swellings or vesicles and are thus often termed vesicular-arbuscular mycorrhizae. In both cases of symbiosis the fungus supplies the plant 'partner' with water and mineral nutrients, especially if these are in short supply in the soil; whereas the plant supplies the fungus with carbohydrates from photosynthesis.
Fossil Record
Fossil fungi tend to be microscopic and not always found with their reproductive structures attached, therefore positive identification is often extremely difficult. They are not especially rare as fossils, though the best preserved examples are generally found in amber, for example the Oligocene Baltic ambers and Cretaceous ambers from northern France.
The earliest record of fungi in the fossil record are of probable chytridiomycetes or chytrids from Vendian strata (Late Precambrian, 650 to 544 million years ago) of northern Russia. The Early Devonian Rhynie chert is host to a plethora of fossil fungi including various endotrophic mycorrhizae, the earliest ascomycetes, several chytridiomycetes and various other undescribed fungi (see Kidston & Lang 1921b; Hass & Remy 1992; Taylor et al. 1992a & b; Hass et al. 1994; Remy et al. 1994a & b; Taylor et al. 1994, 1995, 1999, in press).
For the purposes of this resource, the morphology and palaeoecology a small selection of different types of fungi from the chert are outlined below:
Morphology
Endotrophic Mycorrhizae


These fungi were terrestrial symbionts, though in part may also have been saprophytic after the death of the plant partner.Ascomycetes

These ascomycetes were terrestrial and probably saprophytic.
The formal description and diagnosis for the fossil ascomycete is currently awaiting publication (Taylor et al. in press).

Chytridiomycetes
The tiny, predominantly aquatic chytrids are the most common forms of fungi found in the Rhynie chert. Most resemble fungi from either of two extant orders, the Blastocladiales and the Spizellomycetales. Both orders include saprophytic and parastic fungi, though living spizellomycetales are mostly found in damp and water-logged soil.



This chytrid was aquatic and a saprophyte.
Other Rhynie chert chytrids were clearly aquatic parasites, three types have been described as parasites on the probable charophyte Palaeonitella cranii by Taylor et al (1992). Below are images of two of these, Milleromyces rhyniensis (inset below left) and Lyonomyces pyriformis (inset below right).
A review of the Rhynie chert fungi is currently awaiting publication (Taylor et al. in press).


Lichens

Introduction
The earliest known fossil lichen, Winfrenatia reticulata (Taylor et al. 1995, 1997) has been described from the Rhynie chert.
Lichens are a group of non-vascular plants that are formed by a symbiotic association between a fungus (termed a mycobiont) and green alga or a cyanobacterium (termed a photobiont). The relationship is mutually beneficial since the photobiont can obtain water and nutrients as well as some degree of protection from the fungus whereas the mycobiont gains a source of carbon. Today lichens are relatively diverse and have adapted to a number of different habitats, for example, being found in mountain, desert and tundra regions.


Fossil Record
Lichens are not common in the fossil record. This partly reflects the fact that the habitats in which they are often found, are rarely conducive to fossil preservation. A few fossil lichens have been described from Mesozoic and Cenozoic rocks, most notably from Oligocene Baltic amber (Larson 1978; Garty et al. 1982). It has been suggested that a number of the Precambrian Ediacara fossils may represent lichens (Retallack 1994) though this remains rather suspect. It is evident that complex microbial communities were present even 3,500 million years ago, however, whether any of these comprised physiological symbioses is uncertain. Thus Winfrenatia reticulata, from the Rhynie chert is considered the oldest known lichen. The morphology of Winfrenatia is outlined below.
Morphology

The surface of the lichen exhibits a series of small pockets that contain an open meshwork or 'net' of 1-4μm diameter fungal hyphae (see insets right and below left). Each 'net' is about 25μm in diameter and contains a single cell or cluster of daughter cells that represent the photobiont (see inset below right). The size of cells and number of daughter cells in the hyphal nets generally increases towards the top of the thallus. Each cell is coated with a relatively thick mucilaginous envelope.


Palaeoecology
As with modern cyanolichen equivalents, during the early Devonian at Rhynie Winfrenatia was most likely an early coloniser of hard substrates. Degrading sinter surfaces could have provided a suitable substrate. Similarly it may have been able to weather the rock surfaces it was colonising and thus contributing to soil formation.
The presence of a cyanolichen in the early Devonian is thus important in understanding the ecology of early terrestrial communities as well as the evolution of land plants.
