
B.Sc. (Hons) in Pharmacology, University of Glasgow 1988, Ph.D., University of Glasgow 1991
Personal Chair
- About
-
- Email Address
- g.f.nixon@abdn.ac.uk
- Telephone Number
- +44 (0)1224 437405
- Telephone Number
- +44 (0)1224 274271
- Office Address
Office - Room 6.31,
University of Aberdeen,
Institute of Medical Sciences,
School of Medicine, Medical Sciences and Nutrition
Foresterhill
Aberdeen
AB25 2ZD
Scotland
United Kingdom
- School/Department
- School of Medicine, Medical Sciences and Nutrition
Biography
Graeme Nixon is the Director of the Scottish Graduate School of Social Science, the UK's largest social science doctoral training partnership funded by the Economic and Social Research Council. He also holds a personal chair in the Institute of Medical Sciences where his research focusses on cardiovascular health and disease.
He completed his PhD degree in Medical Sciences at the University of Glasgow. This was followed by a 4 year American Heart Association postdoctoral fellowship at the University of Virginia before moving to the University of Aberdeen.
Graeme co-founded the Aberdeen Cardiovascular & Diabetes Centre in 2018. It consists of approximately 30 senior laboratory scientists and clinicians from the University of Aberdeen and NHS Grampian working together to improve patient outcomes through research and education in cardiovascular diseases and diabetes. He was also previously the Dean of Postgraduate Research at the University of Aberdeen from 2018-2023, a cross-institutional role with strategic oversight of PhD recruitment and training.
Qualifications
- BSc Pharmacology1988 - University of Glasgow
- PhD Medical Sciences1991 - University of Glasgow
Latest Publications
An endogenous inhibitor of angiogenesis downregulated by hypoxia in human aortic valve stenosis promotes disease pathogenesis
Journal of Molecular and Cellular Cardiology, vol. 174, pp. 25-37Contributions to Journals: ArticlesPhosphoprotein enriched in astrocytes (PEA)-15 is a novel regulator of adipose tissue expansion
Scientific Reports, vol. 11, 6949Contributions to Journals: ArticlesPEA‐15 (Phosphoprotein Enriched in Astrocytes 15) Is a Protective Mediator in the Vasculature and Is Regulated During Neointimal Hyperplasia
Journal of the American Heart Association, vol. 6, no. 9, e006936Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1161/JAHA.117.006936
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/9294/1/e006936.full.pdf
Sphingosylphosphorylcholine inhibits macrophage adhesion to vascular smooth muscle cells
Biochemical Pharmacology, vol. 115, pp. 43-50Contributions to Journals: ArticlesThe cAMP-producing agonist beraprost inhibits human vascular smooth muscle cell migration via exchange protein directly activated by cAMP
Cardiovascular Research, vol. 107, no. 4, pp. 546-555Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1093/cvr/cvv176
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstream/2164/4659/3/546.full.pdf
- Research
-
Research Overview
My research interests are predominantly in the development of new therapeutics for cardiovascular disease. In addition, I am also interested in research training strategies for the next generation of medical researchers to ensure impact and clear societal value are embedded in research design.
In particular, current areas of focus are:
1) the link between obesity and cardiovascular disease, and specifically drug targets to modulate lipid storage to slow disease progression.
2) uncovering new drug targets for aortic valve stenosis
Research Areas
Accepting PhDs
I am currently accepting PhDs in Biomedical Sciences.
Please get in touch if you would like to discuss your research ideas further.
Current Research
Modulating lipid storage in obesity to prevent cardiovascular disease
While the link between obesity and cardiovascular disease is well-defined, the relationship between fat storage and the development of cardiovascular disease is less clear. Fat storage in adipose tissue of non-obese individuals is physiologically "safe" and important for normal metabolic health. An excessive calorific intake results in expansion of adipose tissue which, if continued, leads to lipid distribution to areas outside the adipose tissues, such as the liver, heart and blood vessels. This is physiologically "unsafe" and is the trigger for development of metabolic and cardiovascular diseases. This tipping point between "safe" and "unsafe" lipid storage is the key to understanding metabolically dependent cardiovascular disease.
Our research is examining new therapeutic targets to improve the "safe" storage of lipids in adipose tissue and prevent the overspill of lipids into other non-adipose tissues. Recently, we have uncovered a protein present in adipocytes (the cell type responsible for lipid storage in adipose tissue) which can regulate lipid storage capacity. This protein is called phosphoprotein enriched in astrocytes-15 (PEA-15). In mice which do not express the PEA-15 protein, they can continue to expand their adipose tissue and store for lipids safely while decreasing unsafe lipid storage. This directly reduces cardiovascular disease in this model (Veschoor et al ,2021).
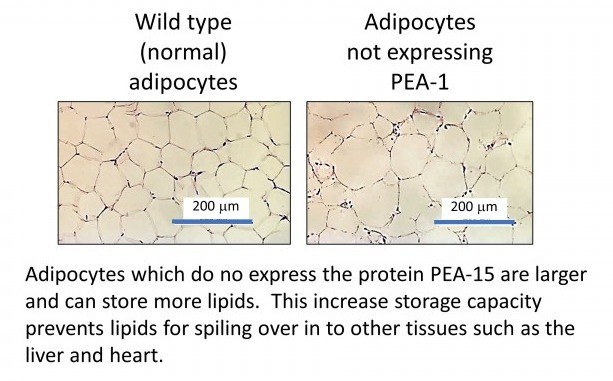
We have also demonstrated this in human adipose tissue in the laboratory.
Video to explain this research for a non-scientific audience
Uncovering new therapeutic targets for aortic valve stenosis
The aortic valve is situated at the exit point where blood leaves the heart during contraction of the heart muscle and enters the blood vessel (initially the largest artery known as the aorta). It's innate flexibility allows the valve to open during heart contraction allowing minimal disruption to flow and quickly close following this, thereby preventing a reversal of blood flow back in to the heart. In patients who develop aortic valve stenosis, the valves undergo a change in structure which involves a calcification within the valve wall - this is similar to the formation of bone-like structures (calcified nodules, see figure below). These nodules are brittle and inflexible, leading to decreased efficiency of valve function. Blood does not flow as easily out of the heart leading eventually to heart failure, unless treated. Current treatment consists of surgery to replace the defective heart valve with a prosthetic valve.
Aortic valve stenosis is associated with cardiovascular risk factors and is closely linked with old age. It is becoming increasingly common amongst the elderly population and the early stages of valve calcification is currently thought to be present 70% of the population aged 65 years and over. Despite this significant clinical problem, there are currently no drug treatments which can prevent or slow the progression of aortic valve stenosis.
Our research is directed towards identifying new therapeutic targets to slow the progression of aortic valve stenosis (Lewis et al, 2023)
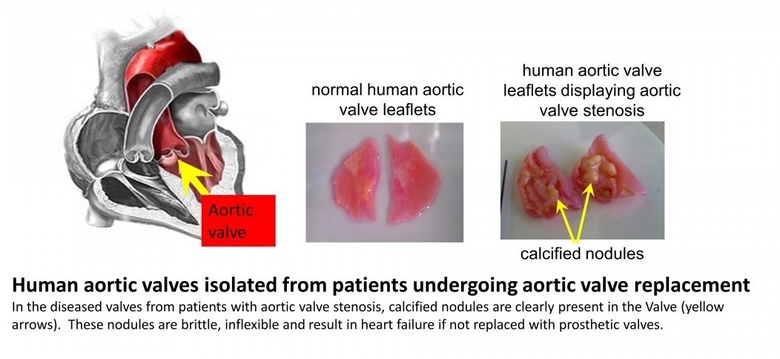
Past Research
Dietary zinc deficiency and blood vessel function
The micronutrient zinc is critical to many physiological processes in vivo and has an essential role in health. Dietary zinc deficiency, which occurs due to inadequate intake, decreased absorption or increased loss can therefore be an important factor in many pathological conditions. This is particularly relevant in the elderly where zinc utilization and absorption are known to be decreased with age. Recently, evidence has emerged suggesting that zinc deficiency is associated with the development of cardiovascular diseases such as atherosclerosis. The cell types and intracellular mechanisms involved in these zinc-dependent atherogenic changes are not known and could be due to effects on vascular smooth muscle cells. Our recent research has now indicated that dietary zinc deficiency results in programmed cell death (apoptosis) in the vascular smooth muscle cells. This includes depolarized mitochondria, a hallmark of the apoptotic process (see image below). Apoptosis has a role in the clinical problems associated with atherosclerosis.

This research indicates that those with lower zinc intake (such as the elderly) may exacerbate underlying atherosclerosis and advocates adequate zinc to protect against cardiovascular disease.
Regulating the growth of new coronary arteries in the human heart
We have developed a model of "human coronary arteries in a dish". This model uses the cell types that normally constitute a human coronary artery in the heart. By combining these cell types and treating with specific growth factors, we can induce development of a coronary artery blood vessel networks. Using this novel approach, we have shown that an endogenous lipid known called sphingosine 1-phosphate blocks the growth of new coronary arteries by preventing tubule formation of the endothelial cells (see image below). Our paper (Mascall et al, 2012) details the mechanisms of this inhibitory effect.

Collaborations
Prof. Joe Ramos, University of Hawaii, USA
Prof A. Mark Evans, University of Edinburgh, UK
Dr. Justin Rochford, University of Aberdeen, UK
Prof. Mirela Delibegovic, University of Aberdeen, UK
Supervision
My current supervision areas are: Biomedical Sciences.
Current PhD students:
Giovanni Levate: Oct 2019 - Sept 2022 "Novel protein regulators of adipocyte lipid storage"
Completed PhD students (last 10 years):
Sarah Zhang: Oct 2017-Sept 2021
Christopher Lewis: Oct 2017 – Sept 2020
Pola Verschoor: Oct 2016 – Sept 2019
Jenny McKean: Oct 2011 – Jan 2015
Keith Allen-Redpath: Aug 2009 – Dec 2012
Keith Mascall: Oct 2008 – July 2013
- Teaching
-
Teaching Responsibilities
Teaching across level 1-4 at undergraduate and at level 5 (MSc).
Undergraduate Year 1:
SR1002: Introduction to the science of sport, exercise and health
Undergraduate Year 2:
SM2001: Foundation skills for medical sciences
Undergraduate Year 3:
PA3004: Biochemical pharmacology and toxicology
BM3501: Cardiovascular physiology and pharmacology
Undergraduate Year 4:
BM4009: Staying alive: the physiology of adaptation*
BM4004: Advanced membranes, molecules and cells
PA4005: Molecular pharmacology
Other:
BDS2 (dental students): Introduction to blood vessels
MSc: small molecule drug discovery
*course coordinator
- Publications
-
Page 4 of 4 Results 31 to 36 of 36
Expression and distribution of InsP(3) receptor subtypes in proliferating vascular smooth muscle cells
Biochemical and Biophysical Research Communications, vol. 273, pp. 907-912Contributions to Journals: ArticlesETA receptors are the primary mediators of myofilament calcium sensitization induced by ET-1 in rat pulmonary artery smooth muscle: a tyrosine kinase independent pathway
British Journal of Pharmacology, vol. 127, pp. 153-160Contributions to Journals: ArticlesExpression and distribution of the type 1 and type 3 inositol 1,4,5-trisphosphate receptor in developing vascular smooth muscle
Circulation Research, vol. 84, pp. 536-542Contributions to Journals: ArticlesTumour necrosis factor-alpha activates a calcium sensitization pathway in guinea-pig bronchial smooth muscle
The Journal of Physiology, vol. 518, pp. 561-569Contributions to Journals: ArticlesLocalization of ryanodine receptors in smooth muscle
Circulation Research, vol. 82, no. 2, pp. 175-185Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1161/01.RES.82.2.175
- [ONLINE] View publication in Scopus
Increased sensitization of the myofilaments in rat neonatal portal vein: A potential mechanism
Experimental Physiology, vol. 82, no. 6, pp. 985-993Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1113/expphysiol.1997.sp004084
- [ONLINE] View publication in Scopus
